A Study on the Bacteriological Profile and Antibiogram of Bacteremia in Children Below 10 Years in a Tertiary Care Hospital in Bangalore, India
Devendra Kumar Tiwari1, Saroj Golia2, Sangeetha K.T.3, Vasudha C.L.4
1 Tutor, Dr. B.R Ambedkar Medical College, Bangalore, India.
2 Professor & HOD, Dr. B.R Ambedkar Medical College, Bangalore, India.
3 Postgraduate Student, Dr. B.R Ambedkar Medical College, Bangalore, India.
4 Postgraduate Student, Dr. B.R Ambedkar Medical College, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sangeetha K.T., TF-04, Sneha Sindhu Apartments, Shampura Main Road, Near Dr. B.R Ambedkar Medical College, Kavalbyrasandra, R.T. Nagar Post, Bangalore, Karnataka-560032, India.
Phone: 7829222120,
E-mail: ktsan85@gmail.com
Introduction: Blood stream infections are very common in the pediatric age group. Patients with bacteremia may have either a transient bacteremia that may be rapidly and permanently cleared by a patient’s host defenses with no major consequences, or persistent bacteremia which can be self-limited without development of focal infection or sequelae, or may progress to a more serious fatal infection or toxic symptoms.
Objectives: The aim of our study is to analyze the hospital data on bacteremia in children less than 10 years with special reference to male and female cases, the pathogens involved, and the antibiotic susceptibility patterns.
Methods: Over a one year period samples were collected from 128 children who included all newborn babies and children admitted with fever and suspected of having sepsis. Blood was collected depending upon age groups with aseptic precaution and incubated at 37oC for 10 days. Subcultures were made on blood agar and MacConkey agar plates. Organisms were identified and antibiotic sensitivity test of the isolates were performed.
Results: Out of 128 suspected cases, 32 (25%) was culture positive. Male to female ratio is 1.28:1.0. Klebsiella species (43.75%) was the most common organism isolated followed by Staphylococcus aureus (18.75%). Prevalence of gram negative organism was 71.87%. Most of the gram negative organisms showed maximum resistance to ampicillin and the gram positive organisms to penicillin. In this study three gram negative organisms were extended-spectrum beta lactamases (ESBLs) producers and one Pseudomonas aeruginosa was metallo-beta lactamase (MBL) producer. 33.33% of staphylococcus aureus was Methicillin resistant Staphylococcus aureus (MRSA) strains.
Interpretation and Conclusion: This study showed a 25% prevalence rate of bacteremia among children with an increasing prevalence in the age group of 5-10 years and also an observed decline in susceptibility of the pathogens to common antibiotics which ultimately stresses on the need for continuous screening and surveillance for antibiotic resistance in the pediatric care unit and calls for increased efforts to ensure more rational use of these drugs.
Bacteremia, Klebsiella, Staphylococcus aureus, Extended-spectrum beta lactamases (ESBLs), Methicillin resistant Staphylococcus aureus (MRSA)
Introduction
Bacteremia signifies the presence of bacteria in the blood stream [1]. Bacteremia may be transient, continuous or intermittent. Microorganisms present in the circulating blood, whether continuously, intermittently, or transiently, are a threat to every organ in the body. They can have serious consequences like shock, multiple organ failure, disseminated intravascular coagulation, etc. Thus, the blood stream infections constitute one of the most serious situations and, as a result, timely detection and identification of blood stream pathogen is important [2]. Blood culture plays an integral role in the evaluation of sepsis [3].
Neonates are particularly vulnerable to infections because of their weak immune barrier. Several risk factors have been identified both in the neonates and children which makes them susceptible to infections [4]. Children with septicaemia present with fever, difficulty in breathing, tachycardia, malaise, refusal of feeds or lethargy [5].
A relative number of bacterial species causes the vast majority of bacteremia in normal children. Streptococcus pneumoniae, Haemophilus influenzae type B and Neisseria meningitidis are among the most common isolates and each may be associated with occult bacteremia as well as severe sepsis. Staphylococcus aureus, Salmonella species and Group Astreptococci are also pathogenic that may be isolated from blood cultures in children who usually have moderate or severe illness. In otherwise normal children gram negative enteric species may cause bacteremia in association with pyelonephritis or diarrhea. The presence of foreign material such as catheter or central line enhances the risk of bacteremia with both gram positive (Coagulase negative Staphylococci, Staphylococcus aureus, and Streptococcus species) and gram negative bacteria [6].
In healthy and immunocompetent host, a sudden infuse of bacteria is usually cleared from the blood within 30 to 45 minutes. The liver and spleen play the primary role in clearing bacteria; intravascular neutrophils play only a minor role. Septicemia is a clinical syndrome characterized by fever, chills, malaise, tachycardia, hyperventilation and toxicity or prostration, which results when circulating bacteria multiply at a rate that exceeds removal by phagocytes [7].
The successful recovery of microorganism from blood by possible types of bacteremia depends upon specimen collection methods, blood volumes, the number and timing of blood cultures, interpretation of results and the type of patient’s population being served by the laboratory [2]. There is a wide variation in the incidence and clinical characteristics of invasive infections caused by different species of bacteria. Identifying the causative agents and characterizing the clinical significance in a particular age group is essential for the prevention and treatment of these infections [8].
As bacteremia continues to be a serious problem that needs immediate attention and treatment, the aim of this study was to determine the causative agents of bacteremia in children below 10 years and their susceptibility to the commonly used antibiotics.
Material and Methods
Blood Samples
In this study 128 blood samples were collected from children (aged from 1day to 10 years) admitted to the paediatric ward of Dr. B.R. Ambedkar Medical College, Bangalore, India during a period of one year (2009 to 2010). The patients included all newborn babies and children admitted with fever and suspected of having sepsis. Children with fever less than 5 days and with known clinical condition such as malignancies, tuberculosis etc. were excluded.
The cases were categorized into 4 clinical groups: Group I [0-1 month–neonates], Group II [1 month-1 year old], Group III [1year-5 years old] and Group IV [5 year -10 years].
Blood for culture was collected from 128 clinically diagnosed septicemia cases following strict aseptic precautions. One milliliter (neonates) and 5 ml (children) blood was collected and inoculated into 10 and 50 ml, respectively, of brain heart infusion broth (1:10 dilution). The culture bottles were incubated at 37°C aerobically and periodic subcultures were done onto Mac Conkey’s agar, blood agar and chocolate agar after overnight incubation on day 3, day 4 and finally on day 7 [9].The growth obtained was identified by conventional biochemical tests and the antibiotic sensitivity testing was performed on Mueller–Hinton agar plates by Kirby–Bauer disc diffusion method. Zone diameter was measured and interpreted as per the Clinical and Laboratory Standards Institute (CLSI) guidelines [10].
Bacterial sensitivity was tested for the following antimicrobials: Amikacin, Amoxicillin-Clavulanic acid, Ampicillin, Aztreonam, Cefotaxime, Ceftazidime, Ceftriaxone, Cephalexin, Cefoxitin, Ciprofloxacin, Gentamicin, Imipenem, Meropenem, Piperacillin-tazobactam, Tobramycin, linezolid and Vancomycin.
Methicillin resistance in Staphylococcus aureus (MRSA)was tested using Mueller-Hinton agar with 4% NaCl with cefoxitin disc (30 micrograms) by Kirby-Bauer disc diffusion method. A zone size of >22 mm was considered sensitive and < 21 was considered resistant [10]. Suspected extended-spectrum beta lactamases (ESBLs) producing organisms were confirmed by double disk synergy test as described previously [11]. Detection of plasmid-mediated AmpC was done by the AmpC disk test and the isolates showing reduced susceptibility to carbapenems (imipenem and meropenem) were selected for detection of metallo-beta lactamases (MBLs) enzymes by imipenem-EDTA disk method [12]. For quality control of disc diffusion tests ATCC control strains of E. coli ATCC 25922, S. aureus ATCC 25923 and P. aeruginosa ATCC 27853 strains were used.
Statistical Analysis
The results were expressed as percentages for the analysis of various epidemiological details and for analysing the distribution of different bacterial isolates and their sensitivity pattern. Microsoft excel was used for the interpretation of these results.
Results
A total of 128 blood samples of which 67(52.34%) were from males and 61(47.66%) from females were subjected to culture. Bacteremia was more common in the age group of 5-10 years (35.71%) [Table/Fig-1] and was more frequently seen in male children (26.87%) [Table/Fig-2]. Out of 128 samples, 32 samples (25%) were culture positive and all of them showed monobacterial growth. None of the blood samples yielded polymicrobial growth.
Incidence of Bacteremia in Children according to age group
| Age group | No. of cases Investigated | Bacteremia |
|---|
| Number | Percentage |
|---|
| 0-1 month | 53 | 12 | 22.64% |
| 1 month–1 year | 34 | 6 | 17.65% |
| 1-5years | 27 | 9 | 33.33% |
| 5-10years | 14 | 5 | 35.71% |
| Total | 128 | 32 | 25% |
Sex distribution among bacteremia cases
| Sex | Total no. of cases investigated | Positive blood culture |
|---|
| Male | 67 | 18 (26.87%) |
| Female | 61 | 14 (22.95%) |
[Table/Fig-3] and chart 3 describes the distribution of the total bacterial isolates obtained in the positive blood cultures. Out of total 32 positive cultures, Klebsiella species was the predominant organism isolated 43.75% (14/32), followed by Staphylococcus aureus 18.75% (6/32) and Pseudomonas aeruginosa 9.38% (3/32) and CONS 9.38% (3/32). 11 out of 14 Klebsiella species were Klebsiella pneumonia and the remaining Klebsiella oxytoca.
Distribution of Organisms Isolated from Blood culture
| Name of Organism | Number | Percentage |
|---|
| Klebsiella species | 14 | 43.75 |
| Staphylococcus. aureus | 6 | 18.75 |
| Coagulase Negative staphylococci (CONS) | 3 | 9.38 |
| Pseudomonas aeruginosa | 3 | 9.38 |
| Salmonella typhi | 2 | 6.25 |
| E.coli | 2 | 6.25 |
| Acinetobacter baumanii | 1 | 3.13 |
| Citrobacter freundii | 1 | 3.13 |
| Total | 32 | 100.00 |
[Table/Fig-4] shows the distribution of gram negative organism to be 71.87% as against 28.13% of gram positive organism.
Prevalence of Gram Negative and Gram positive Bacterial Isolates
| Organism | Number | Percentage |
|---|
| Gram Negative | 23 | 71.87% |
| Gram Positive | 09 | 28.13% |
| Total | 32 | 100.0 |
Discussion
The varying microbiological pattern of bacteremia in children warrants the need for an ongoing review of the causative organisms and their antimicrobial susceptibility pattern [13].
In our study, it was observed that the incidence of bacteremia was higher in males compared to females. Nimri et al., [8] and Joshi et al., [14] also observed a higher incidence of bacteremia in males. Bacteremia was more common in the age group of 5-10 years (35.71%) which was in contrast to studies done by Tsering et al., [15] and also Meremkwer et al., [4] who reported that bacteremia was most frequently encountered in newborns.
Out of the 128 clinically suspected cases of sepsis in our study, 32 were culture positive with a blood culture positivity rate of 25%. Similar positivity rates were reported by other studies also [13,15]. Higher positivity rates of (43.78%) have been observed by Prabhu K et al., [9].
Gram-negative septicemia was encountered in 71.87% of the culture positive cases. This is in concordance with studies done by Ali Z et al., (63%) [16] and Sharma M et al., (72.7%) [13].
Klebsiella (43.75%) species was the commonest isolate associated with bacteremia in our study. Al-Charrakh et al., [17] also reported a high incidence of Klebsiella septicemia (46.8%). The next commonest isolate obtained was Staphylococcus aureus (18.75%). Gram positive organisms mainly Staphylococcus aureus have been shown as the most frequently isolated bacteria causing bacteremia by many studies [9,18]. The frequency of infection by various organisms varies from one institution to another institution and even year to year in the same institution [13].
Most of the gram negative organisms showed maximum resistance to ampicillin (68.42%) [Table/Fig-5]. Similar findings have also been observed by Prabhu K et al., (64.28%) [9]. Out of the 23 gram negative isolates three (2 klebsiella spp and 1 E.coli) were ESBL producers. Most of the gram negative bacterial isolates were sensitive to imipenem and meropenem. Among the non-fermenters, 33.33% of Pseudomonas species showed resistance to imipenem and meropenem which is in concordance with studies done by Rahbar et al., (40%) [19]. One of the Pseudomonas species isolate was an MBL producer.
Antibacterial resistance pattern of the Gram negative blood stream isolates
| Antibiotics | Klebsiella spp (n=14) | E.coli (n=2) | Pseudomonas aeruginosa (n=3) | Acinetobacter baumanii (n=1) | S.typhi (n=2) | Citrobacter freundii (n=1) |
|---|
| Ampicillin | 9(64.28%) | 100% | NT | NT | 1(50%) | 100% |
| Amoxyclav | 2(14.29%) | 0 | NT | NT | 0 | 0 |
| Amikacin | 2(14.29%) | 1(50%) | 1(33.33%) | 0 | 0 | 0 |
| Cotrimoxazole | 6(42.86%) | 1(50%) | NT | 0 | 1(50%) | 0 |
| Gentamycin | 2(14.29%) | 1(50%) | 2(66.66%) | 0 | 0 | 0 |
| Tobramycin | NT | NT | 1(33.33%) | 0 | NT | NT |
| Ciprofloxacin | 5(35.71%) | 1(50%) | 2(66.66%) | 0 | 0 | 0 |
| Cefotaxime | 2(14.29%) | 1(50%) | NT | NT | 0 | 0 |
| Ceftriaxone | 2(14.29%) | 1(50%) | NT | NT | 0 | 0 |
| Ceftazidime | NT | NT | 1(33.33%) | 0 | NT | NT |
| Piperacillin- tazobactum | NT | NT | 1(33.33%) | 0 | NT | NT |
| Aztreonam | 3(21.43%) | 1(50%) | 2(66.66%) | 0 | NT | 0 |
| Imipenem | 0 | 0 | 1(33.33%) | 0 | 0 | 0 |
| Meropenem | 0 | 0 | 1(33.33%) | 0 | 0 | 0 |
The gram positive organisms showed 77.78% resistance to penicillin but were 100% sensitive to linezolid and vancomycin. Among the 6 Staphylococcus aureus, 2(33.33%) were detected as Methicillin resistant Staphylococcus aureus (MRSA). Studies by Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India reported a 41% prevalence of MRSA [20]. 2 isolates of Salmonella typhi were isolated from children between 5-10 years age group, among which 50% of them were resistant to ampicillin and cotrimoxazole [Table/Fig-6,7].
Percentage of bacterial resistance to various antibiotics
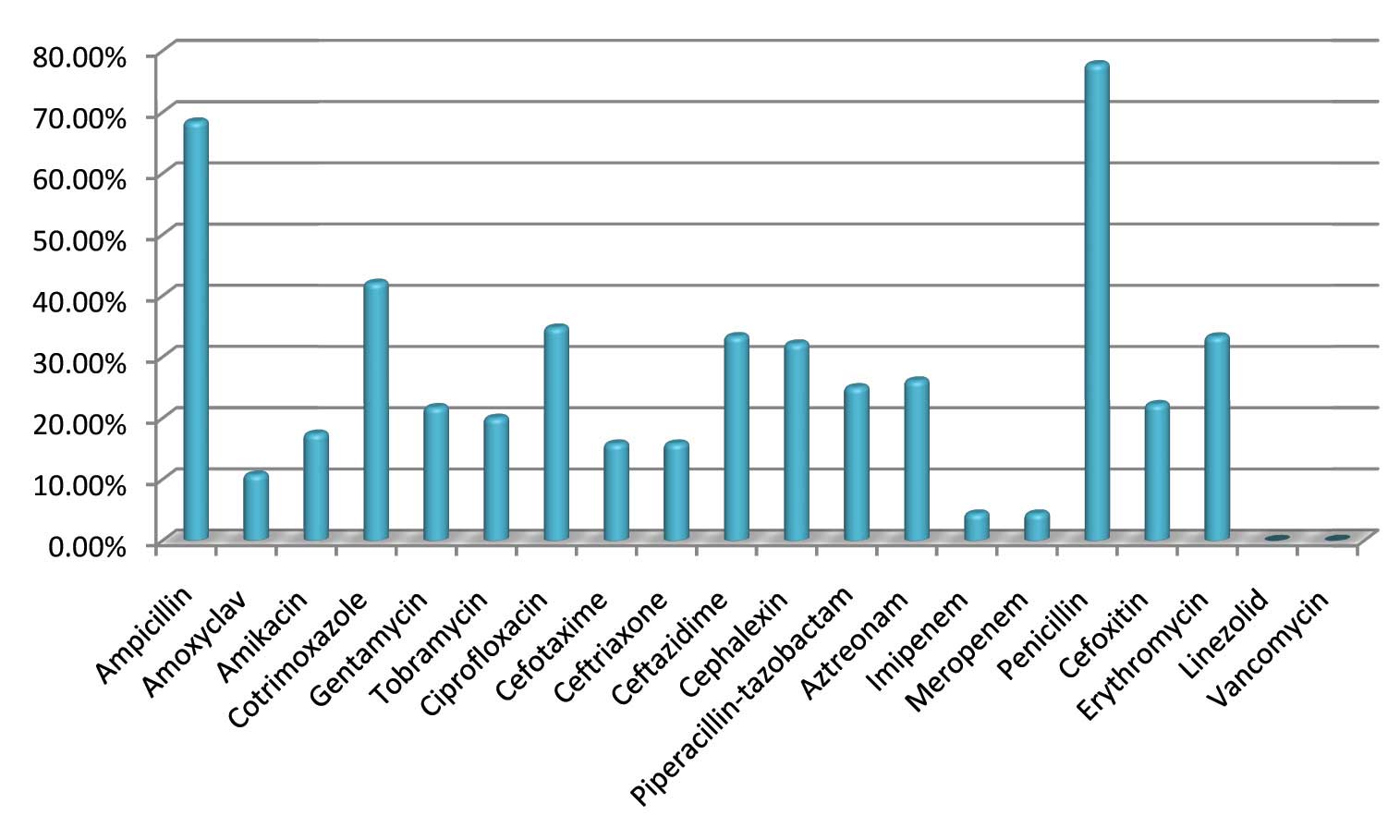
Antibacterial resistance pattern of the Gram positive blood stream isolates
| Antibiotics | Staphylococcus aureus (n=6) | CONS (n=3) |
|---|
| Penicillin | 5(83.33%) | 2(66.66%) |
| Amoxyclav | 1(16.66%) | 0 |
| Cefoxitin | 2(33.33%) | 0 |
| Erythromycin | 2(33.33%) | 1(33.33%) |
| Cephalexin | 2(33.33%) | 0 |
| Linezolid | 0 | 0 |
| Vancomycin | 0 | 0 |
Bacterial infections are the major causes of morbidity and mortality in children. The detection, identification and susceptibility testing of a causative species of bacteria are essential for the proper treatment, and better prognosis of patient. Growing resistance to conventional and even newer antibiotics is a serious cause of concern.
Conclusion
Blood culture still remains as one of the most important microbiological tests available to the clinician for the diagnosis of bacteraemia. The blood culture positivity rate in this study was 25%, with the prevalence being higher among children aged between 5-10 years. In this study carbapenems seem to be a reasonable alternative to the commonly used anti gram negative penicillins as well as to extended spectrum cephalosporin against the Gram negative isolates which turned out to be the major pathogens, except for Pseudomonas species which showed a higher resistance pattern against carbapenems. However a larger sample size study should be performed to validate the findings of the present study. This study emphasizes on the need for continuous screening and surveillance for antibiotic resistance in the pediatric care unit and also in formulation of an antibiotic policy as well as a protocol for the effective management and prevention of drug resistance.
[1]. Campos JM, McNamara AM, Howard BJ, Specimen collection and Processing. In Howard BJ, Keiser JF, Smith TF, Weissfield AS, Tilton RC. EditiorsClinical and Pathogenic Microbiology 1994 2th editionUSAMosby:11.213-11.242. [Google Scholar]
[2]. Forbes BA, Sahm DF, Weissfeld AS, In: Bailey and Scott’s Diagnostic Microbiology 2007 12th edMissouriMosby Elsevier:779 [Google Scholar]
[3]. Shanson DC, Blood culture technique: current controversiesJ Antimicrob Chemother 1990 25(Suppl C):17-29. [Google Scholar]
[4]. Meremkwer MM, Nwachukwu CE, Asuquo AE, Okebe J, Utsalo SJ, Bacterial isolates from blood cultures of children with suspected septicaemia in Calabar, NigeriaBMC Infect Dis 2005 5:110-5. [Google Scholar]
[5]. Nwadioha SI, Nwokedi EOP, Kashibu E, Odimayo MS, Okwori EE, A review of bacterial isolates in blood cultures of children with septicaemia in a Nigerian tertiary HospitalAfr J Microbiol Res 2010 4:222-5. [Google Scholar]
[6]. Baltimore RS, Bogue CW, Infections Disease – Foci of Infection. In: Burg FD, Polin RA, Ingelfinger JR, Gershon AA, EditorsGellis & Karan’s current pediatric therapy 2002 17th EditionUSAElsevier Science:39-256. [Google Scholar]
[7]. Koneman EW, Allen SD, Janda WM, Schreckember PC, Winn WC, Koneman’s Colour Atlas and text book of Diagnostic Microbiology 2006 6th editionNewyorkLippincott:97-99. [Google Scholar]
[8]. Nimri L.F, Ravashdeh M, Meqdam M.M, Bacteremia In Children: Etiologic Agents, Focal Sites, And Risk FactorsJr of tropical pediatrics 2001 vol 47:356-60. [Google Scholar]
[9]. Prabhu K, Bhat S, Rao S, Bacteriologic profile and antibiogram of blood culture isolates in a pediatric care unitJ Lab Physicians 2010 2:85-8. [Google Scholar]
[10]. CLSI – Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing. Twenty-second informational supplement. Wayne, PA, USA: CLSI:2012;M100-S22 [Google Scholar]
[11]. Chaudhary U, Aggarwal R, Extended spectrum-lactamases (ESBL) - An emerging threat to clinical therapeuticsIndian J Med Microbiol 2004 22:75-80. [Google Scholar]
[12]. Noyal MJC, Menezes GA, Harish BN, Sujatha S, Parija SC, Simple screening test for detection of carbapenamases in clinical isolates of nonfermentative gram negative bacilliIndian J Med Res 2009 29:707-12. [Google Scholar]
[13]. Sharma M, Yadav A, Goel N, Chaudary U, Microbial profile of septicemia in childrenInd jr for the practicing doctor Vol 5 (No.4)(2008-09-2008-10) [Google Scholar]
[14]. Joshi SG, Ghole VS, Niphadhar, Neonatal Gram-Negative BacteremiaIndian Journal of Pediatrics 2000 67(1):27-32. [Google Scholar]
[15]. Tsering D C, Chanchal L, Pal R, Kar S, Bacteriological Profile of Septicemia and the Risk Factors in Neonates and Infants in SikkimJ Glob Infect Dis 2011 Jan-Mar 3(1):42-45. [Google Scholar]
[16]. Ali Z, Neonatal bacterial septicaemia at the Mount Hope Women’s Hospital, TrinidadAnn Trop Paediatr 2004 24(1):41-4. [Google Scholar]
[17]. Al-Charrakh Alaa H, Al-Muhana Al-Saadi Zainab, Bacterial Profile of Blood Stream Infections In Children Less Than Three Years OldJ. Babylon Univ 2005 10(30):481-85. [Google Scholar]
[18]. Mohammad A, Bacteremia among Jordanian children at Princess Rahmah Hospital: Pathogens and antimicrobial susceptibility patternsIran J Microbiol 2010 2(1):22-26. [Google Scholar]
[19]. Rahbar M, Monnavar KM, Vatan KK, Fadaei-haq A, Shakerian F, Carbapenem resistance in gram-negative bacilli isolates in an Iranian 1000-bed Tertiary HospitalPak J Med Sci 2008 24(4):537-40. [Google Scholar]
[20]. Joshi S, Ray P, Manchanda V, Bajaj J, Chitnis D.S., Gautam V, Methicillin resistant Staphylococcus aureus (MRSA) in India: Prevalence & susceptibility patternIndian J Med Res 2013 137:363-69. [Google Scholar]