Sclerosing mucoepidermoid carcinoma (SMEC) is a distinct but an uncommon salivary gland neoplasm with only 19 cases reported in English literature till date. Densely collagenous sclerotic stroma, resemblance to other benign lesions and rarity of this tumour often makes the diagnosis of SMEC challenging. Here we report a case of SMEC in a 73–year old female patient suffering from filariasis. Clinical, radiological, fine needle aspiration biopsy, gross and histopathological features are discussed with detailed review of literature and probable pathogenesis.
Manuscript
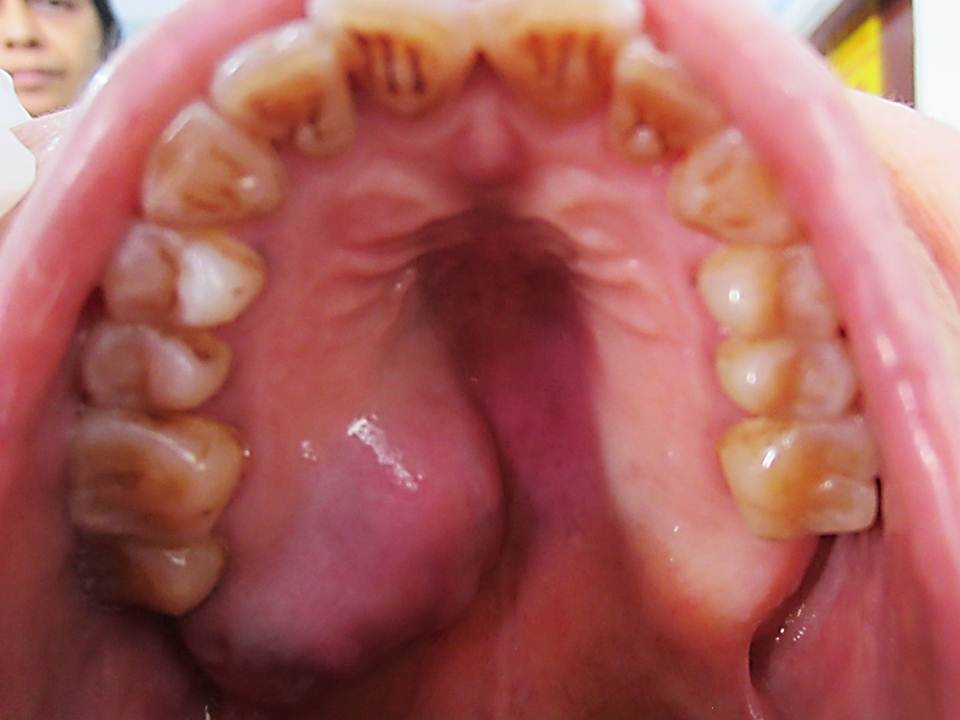
A 73–year-old woman reported with the chief complaint of rapidly enlarging, painless swelling on the right side of the hard palate. Patient first noticed it around two months back had a medical history of uncontrolled hypertension since six years and was suffering from filariasis. She denied any prior orofacial trauma, use of tobacco or alcohol. On physical examination, patient had swellings on both the lower extremities and the overlying skin was normal to erythematous. On head and neck examination, no asymmetry was observed and cervical lymph nodes were not palpable. On extra-oral examination, no swelling was evident. Intra-orally, a swelling of 2.3 x 1.7-cm was observed in relation with the right hard palate with extension to the ipsilateral soft palate [Table/Fig-1]. The swelling extended from the right maxillary permanent 1st premolar region to maxillary tuberosity antero-posteriorly and from palatal gingival margin (in relation with 16, 17) till the palatal midline mesio-distally. It was normal to bluish-red in colour, smooth surfaced without any ulceration or discharge, firm to hard in consistency and fixed to the underlying bone without blanching, pulsation, thrill or bleeding on palpation. No mobility of teeth was observed. Radiographic examination revealed a homogeneous, well delineated radiolucency involving the region associated with the maxillary premolars, molars and tuberosity region but the lamina dura surrounding the roots of the teeth was intact and no root resorption was evident. Although resorption of palatal cortical plate was seen radiographically, no change was noticed in the buccal cortical plate. Patient was kept under close follow up till the formulation of the final diagnosis. However, no medication was prescribed for patient’s chief complaint.
Clinical presentation of the palatal swelling involving the right side of the hard palate
Differential Diagnosis
Multiple benign lesions may appear similar to this patient’s swelling on the palate, including mucocele, haemangioma and pleomorphic adenoma. On examination, the lesion lacked blanching, pulsation, thrill, or bleeding on palpation that negated the possibility of haemangioma. Mucocele also presents with bluish red swelling on the palate, but it is soft on palpation and lacks destruction of the surrounding bone. Filariasis can manifest in oral cavity but the focal swelling, bony destruction and rapid progression of the lesion in the current case rules out its possibility. Alveolar abscess can present as a swelling of the palate but the associated teeth were vital, there was no history of trauma and lamina dura surrounding the teeth roots were intact. Benign tumours like salivary gland adenomas may be considered but they generally lack rapid growth and rather show more gradual enlargement over months to years. Considering the rapid progression of this patient’s lesion over the period of 2 months, malignant neoplasms like mucoepidermoid carcinoma, adenoid cystic carcinoma, polymorphous low grade adenocarcinoma and acinic cell carcinoma were given consideration in formulation of the clinical differential diagnosis.
Diagnosis and Management
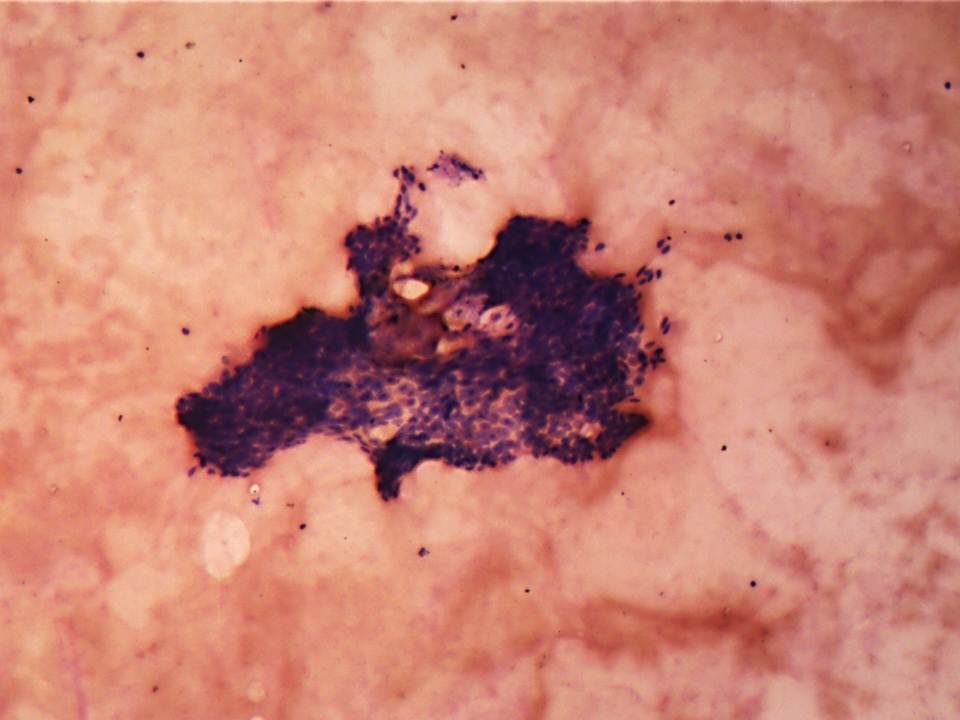
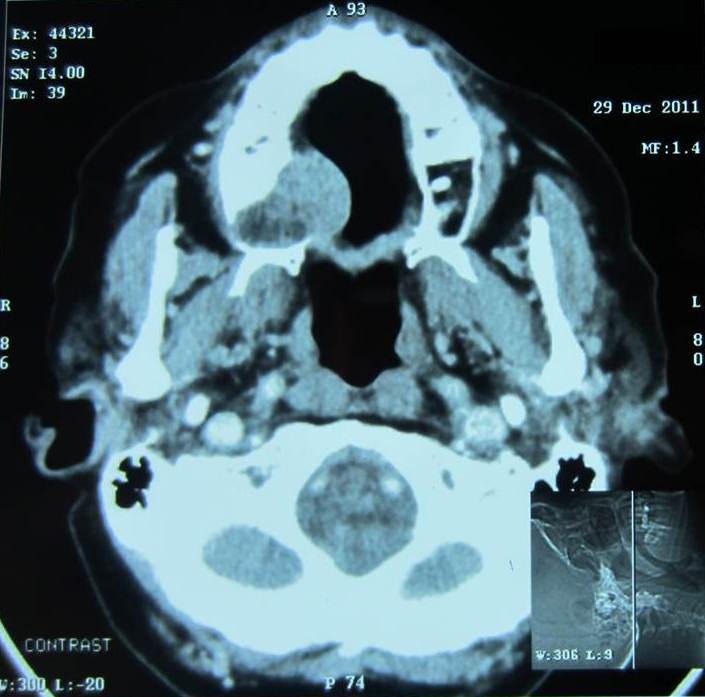
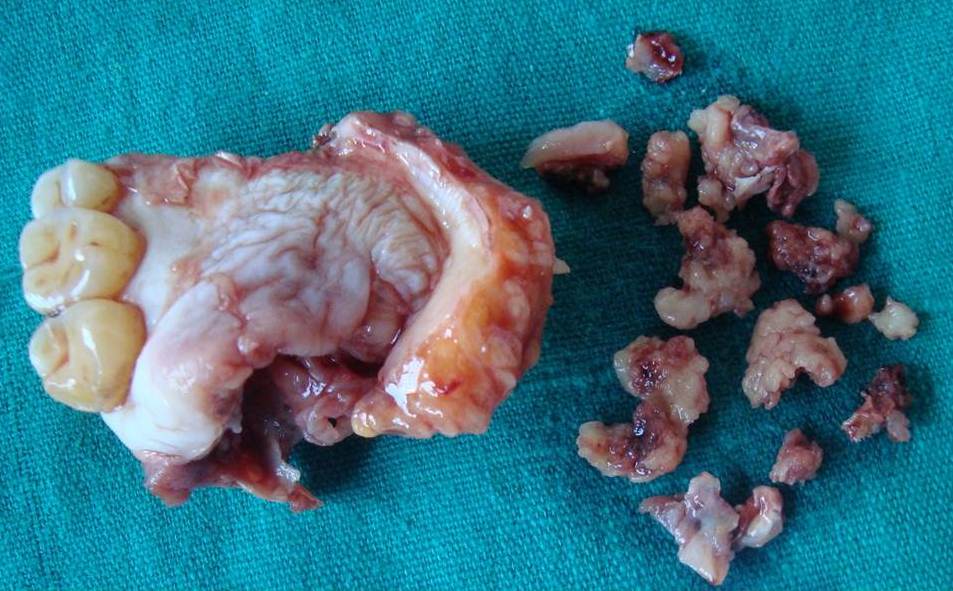
Based on the history, clinical features and radiographic findings, aspiration was recommended. On aspiration blood tinged; odourless, watery to viscous fluid was attained. The cytological smear stained with May Grunwald Giemsa stain showed clusters of multiple basaloid cells with hyperchromatic nuclei lacking cellular atypia and inflammatory cells (chiefly neutrophils and lymphocytes) scattered in the background of RBCs. No evidence of mucin was seen on mucicarmine stained smears [Table/Fig-2]. Computerised tomography (with contrast media) was performed. The axial view showed a well-defined, mildly enhancing mass of soft tissue density in relation to the right side palate [Table/Fig-3]. Bony destruction of the hard palate was evident. Based on the FNAC report, CT scan findings and in view of rapid progression, size and destruction of the surrounding bone by the lesion; working diagnosis of salivary gland tumour arising from minor salivary gland involving hard palate was arrived at. An informed consent was obtained from the patient for the surgical procedure and resection of involved maxilla (subtotal maxillectomy) with clearance was done. The specimen was submitted for histopathological examination. On gross examination, resected right side of the palate with 15, 16, 17 and attached soft tissue was seen [Table/Fig-4].
Cytological smear stained with MGG stain shows clusters of hyperchromaticbasaloid cells scattered in the background of red blood cells. (4x)
CT scan showing well defined mass with soft tissue density. Bony destruction is evident
Gross presentation of the specimen
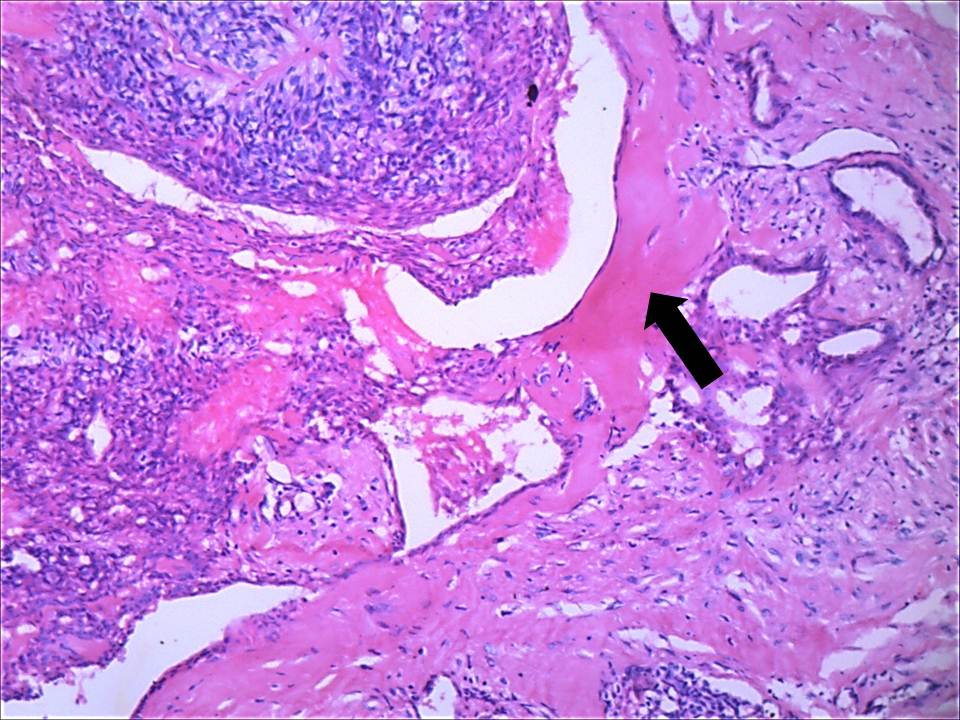
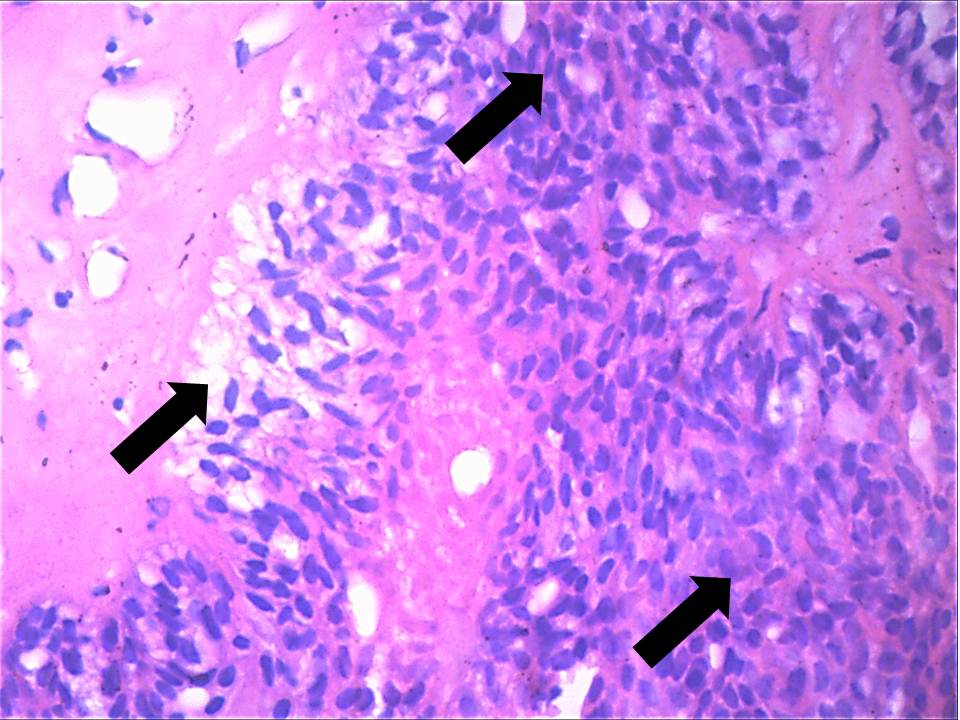
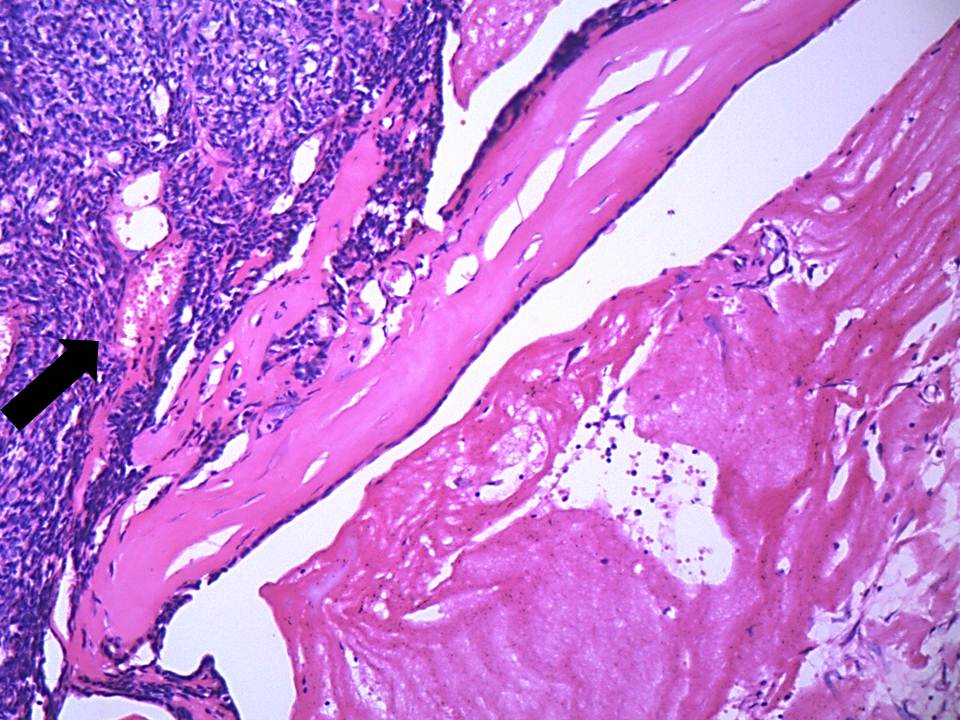
On histologic examination, tissue sections showed dense, hyalinised, sclerotic stroma surrounding the dilated cystic spaces; islands and nests of tumour cells with overlying parakeratinised stratified squamous epithelium. The cystic spaces were filled with PAS and mucicarmine-positive eosinophilic coagulum at focal areas and lined by intermediate cells [Table/Fig-5a]. Sheets and trabeculae of intermediate and clear cells were seen with few interspersed mucicarmine-positive mucous cells. Few epidermoid cells were also seen [Table/Fig-5b]. Vascular invasion [Table/Fig-5c] and invasion of bony trabeculae by tumour cells was evident. Moderate amount of chronic inflammatory cells (chiefly lymphocytes) with areas of haemorrhage and multiple blood vessels were seen. There was no pronounced nuclear atypia, necrosis, perineural spread or mitosis in the specimen. The margins were free of tumour. The final diagnosis of sclerosing mucoepidermoid carcinoma was arrived at based on the histopathological features. The tumour was classified as low grade as per Auclair grading system, and grade III in accordance to Brandwein et al., (due to invasion of bone and blood vessels).The post-operative follow up till date has shown no evidence of recurrence of the lesion.
Cystic spaces lined by intermediate cells with focal areas of eosinophilic coagulum and hyalinised dense bundles of collagen (arrow) are evident (4X, H & E stain)
Arrows indicate clear, intermediate and epidermoid cells with interspersed sclerotic, densely hyalinised stroma (10X, H & E stain)
Vascular invasion is evident by intermediate tumour cells (10X, H & E stain)
Discussion
Chan and Saw described the first case of sclerosing MEC in 1987 [1] and only 19 additional cases have been described since then [Table/Fig-6].
Ninteen cases of sclerosing MEC
| Case No | Author | Age/gender | Location | Size (cm) | Grade | Eosinophils in stroma | Mucin extravasation | Necrosis | Treatment | Follow up |
|---|
| 1 | Chan and Saw, [1] | 36/F | L Parotid gland | 2.2x1.7 | Low | A | | | Partial parotidectomy | Not available |
| 2 | Muller et al., [2] | 17/F | R Parotid gland | 2 | Intermediate | P (focally) | P | A | Partial parotidectomy | Not available |
| 3 | Muller et al., [2] | 60/F | R Parotid gland | 1.5 | Intermediate | P (focally) | A | P | Resection and radiation | Not available |
| 4 | Sinha et al., [3] | 65/M | Minor salivary gland (parapharyngeal space) | 6X5X4 | High | A | P | A | Resection and radiation | Not available |
| 5 | Urano et al., [7] | 57/F | R Parotid gland | 2.5×2 | Low | P | A | A | Partial parotidectomy | Metastasis at 3 years |
| 6 | Urano et al., [7] | 43/M | L Submandibular gland | 4.5×2.5 | Low | P | A | A | Total parotidectomy | Metastases and death at 5 years |
| 7 | Fadare et al., [4] | 44/F | R Parotid gland | 1.5 | Low | A | P | A | Total parotidectomy | NERD for 7 years |
| 8 | Ide et al., [13] | 28/M | Minor salivary gland (oral cavity, retromolar) | 2×2 | Intermediate | A | P | A | Resection | Recurrence after 13 years- high grade tumour (comedo type necrosis) |
| 9 | Heavner S, Shah R, Moyer J, [5] | 23/F | L Parotid gland | 2x1 | Low | P (focally) | A | A | Total parotidectomy and radiation | NERD for 1 year |
| 10 | Lee L, Kwan P et al., [14] | 62/F | L Parotid gland | Not available | High | A | P | A | Superficial parotidectomy | Recurrence in 4 years, 1 cm in size in left parotid- low grade |
| 11 | Hyunchul Kim Ju-Han Lee Eung Seok Lee, et al., [9] | 51/F | L Parotid gland | 1.4×1 | Low | P (occasional) | A | A | Resection and radiation | NERD for 3 months |
| 12 | Veras E, Sturgis E, Luna A, [6] | 70/F | L Parotid gland | 4x3 | Low | P (LP- prominent, germinal centres) | Rare | A | Superficial parotidectomy | NERD for 11 years |
| 13 | Veras E, Sturgis E, Luna A, [6] | 37/M | L Parotid gland | 2.2x1x1 | Low | P (LP- prominent, germinal centres) | Rare | A | Superficial parotidectomy | NERD for 17 years |
| 14 | Veras E, Sturgis E, Luna A, [6] | 49/F | R Parotid gland | 2.6x1.7 | Low | P (LP- prominent, germinal centres) | Rare | A | Superficial parotidectomy | NERD for 4 months |
| 15 | Veras E, Sturgis E, Luna A, [6] | 16/F | L Parotid gland | 2 | Intermediate | P (LP- prominent, germinal centres) | Rare | A | Superficial parotidectomy | NERD for 8 months |
| 16 | Aguiar MC, Bernardes VF et al., [15] | 43/F | Palate (minor salivary gland) | - | - | - | - | - | - | - |
| 17 | Shinhar S, [10] | 57/F | L Parotid gland | 2 | Intermediate | A | A | A | Superficial parotidectomy | NERD for 3 years |
| 18 | Mendelson A et al., [11] | 21/F | L Parotid gland | 1.2x1.5 | Low | A | P | A | Superficial parotidectomy | NERD for 3 years |
| 19 | Kayal L et al., [16] | 24/F | L Palate, minor salivary gland | 4.4x4.2 | Low | A | A | A | Total parotidectomy level II neck dissection | |
| 20 | Present case | 73/F | R hard palate (minor salivary glands) | 6.5x3.5x2 | Low * | A | A | A | Subtotal maxillectomy with clearance | NERD for 6 months |
*Grading done by Auclair’s grading system, A= absent, P= present, NERD: no evidence of recurrence of disease
It has been hypothesised that stromal sclerosis in SMEC is a result of an exaggerated inflammatory response to mucin extravasation resulting in fibrous scarring and the other possibility is sclerosis resulting from silent infarction of the tumour [1].The former theory is more accepted [1–6]. Muller et al., wrote that it is unclear if the stroma is a part of neoplasm or is an existing stroma invaded by neoplastic cells [2]. Urano et al., reported two cases of SMEC with increased eosinophils in the stroma. They termed it as ‘sclerosing mucoepidermoid carcinoma with eosinophilia’. They also suggested that SMEC in which eosinophilic infiltrate is more prominent is associated with good prognosis and eosinophils cause sclerosis [7]. Ide et al., reported the recurrence of a case of SMEC after 13 years of surgical excision which showed solid nests composed exclusively of intermediate cells, with no evidence of stromal sclerosis. They stated that the absence of sclerotic stroma in the recurrent case makes the etiopathogenesis of the SMEC inexplicit. They also raised the suspicion whether SMEC is a specific histologic variant or the sclerosis suggests a nonspecific morphologic pattern [8].
As shown in the [Table/Fig-6], the reported cases (including the present case) have: (1) wide age range of 16-73 years with the mean age of 44.84 years, (2) shows female preponderance (4:1), (3) shows predilection for parotid gland (73.6%), followed by minor and submandibular salivary gland, (4) been of intermediate size (average diameter-2.72 x 2.13cm), (5) are generally of low grade (12 out of 19 cases), 5 cases of intermediate and two cases of high grade have also been reported. Two cases of metastasis to lymph nodes (followed by death in one case) and two cases of recurrence have been reported.
CT and magnetic resonance imaging demonstrate nonspecific, minimally to well enhancing homogenous mass [5,9–11]. Fine-needle aspiration biopsies have failed to yield the diagnosIs of SMEC; and were reported as pathology of unknown type to benign tumour or nondiagnostic [5–7]. This can be due to sclerotic stroma surrounding the neoplastic cells which can be interpreted nondiagnostic [11].
Although most of the reported cases have been classified as low grade, a few cases of aggressive behaviour of tumour by invading nerves [4,5] and muscles [3,5] have been reported. The present case also showed vascular invasion and tumour islands in close proximity to bone. Mucous cells of SMEC stain positive with PAS, mucicarmine and alcian blue stain (pH=2.5). But it may be focally positive in few cases. Immunohistochemically, tumour cells and duct like structures show positivity for cytokeratin and CEA (Urano et al., [7] and Kim H et al.,[9]. Urano et al., examined two cases of SMEC for MIB-1 marker to predict the clinical outcome. They found that MIB-1 staining was higher for the patient who died following the metastasis (7.2%) than in the patient who survived (4.5%) [7]. Veras et al., correlated the expression of MIB-1 with the grading of the tumour and found that the tumour with intermediate grading shows increased expression as compared to those with low grading. Mendelson A et al., reported a case with 5% MIB-1 staining for a low grade SMEC which was consistent with the less aggressive proliferation indices noted earlier [6].
The main differential diagnoses of SMEC are sclerosing polycystic adenosis, chronic sclerosing sialoadenitis, low grade cystadenocarcinoma, pleomorphic adenoma, carcinoma ex pleomorphic adenoma and hyalinizing clear cell carcinoma. Sclerosing polycystic adenosis is differentiated from SMEC by its well-preserved lobular architecture and dilated ducts lined by a bilayered epithelium. Apocrine changes are seen in this epithelium and intermediate, epidermoid, and mucous cells of SMEC are lacking [6]. Also, sclerosing polycystic adenosis has large acinar cells with numerous eosinophilic periodic acid Schiff–positive cytoplasmic granules. These features are absent in SMEC. Chronic sclerosing sialoadenitis (Kuttner’s tumour) shows predilection for the submandibular gland. Histologically, it has preserved lobular architecture, prominent fibrosis and lymphoid hyperplasia and lacks the cystic component lined by the typical cells of MEC [6]. The prominent cystic component of SMEC may resemble low-grade cystadenocarcinoma, but cystadenocarcinoma shows typically papillary-cystic lining. Also, cystadenocarcinoma is devoid of the characteristic intermediate, mucinous, and epidermoid cells of SMEC [6]. Pleomorphic adenoma and carcinoma ex pleomorphic adenoma often show a hyalinised stroma, but can be distinguished from SMEC by an associated presence of myxoid, osseous and chondroid areas [12]. Hyalinising clear cell carcinoma is a rare salivary gland neoplasm characterised by polygonal, glycogen-rich clear cells surrounded by a desmoplastic stroma with conspicuous absence of epidermoid and intermediate cells that are present in MEC [12].
For SMEC of parotid gland, superficial or total parotidectomy with preservation of facial nerve is the treatment of choice depending upon the grade of the tumour. For the tumours of submandibular gland, if the cervical lymph nodes show metastasis, neck dissection is indicated [12]. In the present case of SMEC of minor salivary gland, resection of the involved maxilla along with the tumour was carried out without neck dissection as the clinical evidence of nodal metastasis was lacking.
Conclusion
SMEC is a rare entity chiefly affecting parotid salivary gland. It mimics many benign lesions histologically. The pathogenesis of SMEC is still unclear. Although most of the reported cases are of low histological grading, it may show aggressive behaviour. The prognosis of the tumour depends upon the tumour free surgical margins and the histological grading of the tumour. Close follow up of the patient should be maintained for long term disease control.
*Grading done by Auclair’s grading system, A= absent, P= present, NERD: no evidence of recurrence of disease
[1]. Chan JK, Saw D, Sclerosingmucoepidermoid tumour of the parotid gland: Report of a caseHistopathology 1987 11(2):203-7. [Google Scholar]
[2]. Muller S, Barnes L, Goodurn WJ Jr, Sclerosingmucoepidermoid carcinoma of the parotidOral Surg Oral Med Oral Pathol Oral Radiol Endod 1997 83(6):685-90. [Google Scholar]
[3]. Sinha SK, Keogh IJ, Russell JD, Sclerosingmucoepidermoid carcinoma of minor salivary glands: A case report [letter]Histopathology 1999 35(3):283-84. [Google Scholar]
[4]. Fadare O, Hileeto D, Gruddin YL, Sclerosingmucoepidermoid carcinoma of the parotid glandArch Pathol Lab Med 2004 128(9):1046-49. [Google Scholar]
[5]. Heavner SB, Shah RB, Moyer JS, Sclerosingmucoepidermoid carcinoma of the parotid glandEur Arch Otorhinolaryngol 2006 263(10):955-59. [Google Scholar]
[6]. Veras EF, Sturgis E, Luna MA, Sclerosingmucoepidermoid carcinoma of the salivary glandsAnn Diagn Pathol 2007 11(6):407-12. [Google Scholar]
[7]. Urano M, Abe M, Horibe Y, Sclerosingmucoepidermoid carcinoma with eosinophilia of the salivary glandsPathol Res Pract 2002 198:305-10. [Google Scholar]
[8]. Ide F, Horie N, Shimoyama T, Sclerosingmucoepidermoid carcinoma: a specific histologic variant or nonspecific morphologic patternOral Med Pathol 2010 15:53-55. [Google Scholar]
[9]. Kim H, Lee JH, Lee ES, Sclerosingmucoepidermoid carcinoma of parotid gland-A case reportKorean J of Pathology 2007 41:193-97. [Google Scholar]
[10]. Shinhar SY, Sclerosingmucoepidermoid carcinoma of parotid gland: Case reportEar Nose Throat J 2009 88(11):E29 [Google Scholar]
[11]. Mendelson AA, Macki KA, Chauvin Sclerosingmucoepidermoid carcinoma of the salivary gland: Case report and literature reviewEar Nose Throat J 2010 89(12):600-03. [Google Scholar]
[12]. Ellis G, Auclair P, Mucoepidermoid CarcinomaIn: Atlas of tumor pathology—tumors of the salivary glands, Third series, Fascicle 17 1996 7Washington (DC)Armed Forces Institute of Pathology:155-71. [Google Scholar]
[13]. Ide F, Obara K, Enatsu K, Sclerosingmucoepidermoid carcinoma of oral cavityJ Oral Pathol Med 2005 34:187-89. [Google Scholar]
[14]. Lee LT, Kwan PC, Cheng CH, Sclerosingmucoepidermoid carcinoma of parotid gland with neuralgia: A Case reportClin J Oral Maxillofac Surg 2006 17:101-08. [Google Scholar]
[15]. Aguiar MC, Bernardes VF, A rare case of sclerosingmucoepidermoid carcinoma arising in minor salivary glands with immunohistochemical evaluationMinerva Stomatol 2008 57(9):453-57.(Abstract) [Google Scholar]
[16]. Kayal L, Jayachandran S, Niranzena PA, A rare case of sclerosingmucoepidermoid carcinoma of minor salivary gland– a diagnostic enigmaInt J Dent Case Reports 2011 1(3):43-48. [Google Scholar]