Effect of Vitamin E on Uroepithelial Cells and Changes of Urinary Sediments in Oncology Hospital Nursing Personnel
Rezaei-Basiri Majid1, Hassan Rezazadeh2, Iraj Asvadi-Kermani3, Mahmud Ghazi-Khansari4, Mehri Golchin5, Mojgan Sarmad6
1 Research Scholar, Department of Pharmacology and Toxicology, School of Pharmacy, Tabriz University of Medical Sciences, Tabriz-Iran.
2 Associate Professor, Department of Pharmacology and Toxicology, School of Pharmacy, Tabriz University of Medical Sciences, Tabriz-Iran.
3 Professor, Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz-Iran.
4 Professor, Department of Pharmacology, Faculty of Medicine, Tehran University of Medical Sciences, Tehran-Iran.
5 Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz-Iran.
6 Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz-Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Hassan Rezazadeh, Associate Professor, Department of Pharmacology and Toxicology, School of Pharmacy, Tabriz University of Medical Sciences / Iran.
Phone: +98 - 411 – 3341315, Fax: +98 - 411 – 3344798,
E-mail: rezafar81@hotmail.com
Introduction: Vitamin E is an important natural antioxidant, and its most common and biologically active form is α-tocopherol. The antiproliferative effects of alpha-tocopherol have been previously demonstrated. In this study we investigated the effects of vitamin E on urinary epithelial cells and urinary sediments of nursing from oncology hospital.
Material and Methods: Sixty-two female nursing personnel from oncology hospital participated in the study. They received orally 200mg of vitamin E per day for two weeks. Also prior to vitamin E and after vitamin E administration, the uroepithelial cells counts and other components of urinary sediments were carried out.
Results: There were significant differences in the epithelial cells count and treatment with vitamin E causing significantly more number of epithelial cells and urinary sediments to be excreted in the urine.
Discussion: Vitamin E significantly plays an important role on the excretion of uroepithelial cells and urinary sediments.
Conclusion: In conclusion we propose that use of vitamin E at nontoxic levels would significantly enhance its antioxidative properties, especially among individuals subjected to prophylaxis of occupational hazards.
Nursing personnel, Vitamin E, Urinary sediments, Uroepithelial cells
Introduction
Several studies have performed on biological samples to detect genotoxic effects in humans in connection to their jobs or environment. It is of increasing concern that professional personnel involved in the preparation and administration of antineoplastic drugs are occupationally exposed to these substances which usually causes different body organs disorder or diseases [1–3].
The most requested routine tests are the general urine examination, which covers a chemical analysis (pH, glucose, urobilinogen, etc.), a physical analysis (colour, aspect). The microscopic analysis of urinary sediment in search of formed elements (erythrocytes, leukocytes, bacteria, casts, etc.) [4]. Urine examination has essential diagnostic role in patients with various diseases and provides the physician with very important data to support the diagnosis of several pathologies [5,6]. Additionally, there are vital uroepithelial cells in fresh urine and it has been shown that urinary epithelial cells have vitality function in vitro cell cultures. Given these characteristic differences in the nature of uroepithelial cells and the fact that the majority of adult human cancers are derived from epithelial tissues, the study of epithelial cell in urine has particular relevance toward understanding mechanisms of carcinogenesis in humans [7].
Among many antioxidants, vitamin E has a potential adjuvant in cancer therapy by their ability to induce programmed cell death (apoptosis) [8]. In contrast, tumor cells have been shown to be significantly more sensitive than normal cells to the anticancer effects of vitamin E and cell culture studies have shown that vitamin E significantly inhibits growth and initiates apoptosis in neoplastic cells using treatment doses that have little or no effect on normal cell growth or viability [9,10].
Also anti-oxidative activities of vitamin E stabilize cellular membrane and availability of vital uroepithelial cells in fresh urine and prevents from unsaturated fatty acids oxidation [10].
The aim of present study was to investigate the efficiency of vitamin E as an antioxidant reduce the possible genotoxic risk associated with exposure to antineoplastic drugs on urine epithelial cells activity and urinary sediments of oncology nursing personnel [11].
Material and Methods
Selection of nursing personnel: All 62 healthy and non-pregnant nursing personnel from oncology hospital taking part in this study received detailed information documented by morality committee of the university and filled in consent form concerning the aims of the research study. This article is a part of research project and online available: www.irct.ir [12].
Sampling: Primary samples of all volunteers obtained from morning urine (100 ml) were transferred to laboratory in capped sterile beakers. Then they received orally 200mg of vitamin E daily for two weeks and at the end of 14th day second urine sample (100 ml) were also delivered to laboratory. The samples were maintained in refrigerator for investigation of microscopic urinary sediments.
Reagents: All chemicals used in this study were of analytical grade and obtained from (Merck co, Germany). Vitamin E (dL-alpha-tocopheryl acetate) pearl 200IU purchased from Zahravi pharmaceutical company, Tabriz, Iran.
Preparation of urinary sediments: All the urine samples from the nurse volunteers were centrifuged for 5 minutes using large falcon tubes (2500g). A mixture of 20μl of urinary sediments plus 20μl trypan blue color was used to count epithelial cells by neobar lam and Olympus 30 X of 10 microscope lens. Under microscope, wide epithelial cells with specified nucleus were counted in vital and as well as death forms. Death and vital cells can be distinguished by blue and lemon yellow cytoplasm respectively. Other components of urinary sediments such as urinary casts, mucus, calcium oxalate crystals, uric acid and urates amorph were studied.
Statistical Analysis
The statistical analysis of data was done with SPSS followed by student t-test. The level of significance was chosen at p<0.05.
Results and Observations
In this study, urinary samples of 62 female nursing personnel were evaluated with their consent in before and after orally administration of vitamin E (200 mg). A comparison in urinary epithelial cells count obtained from these two stages is given in [Table/Fig-1].
Uroepithelial cells count of women nursing group (N=62, Age between 25-53)
| Age | Before Vitamin E | After Vitamin E |
|---|
| Death | Vital | Death | Vital |
|---|
| 34 | 26 | 76 | 34 | 105 |
| 41 | 5 | 15 | 6 | 18 |
| 30 | 14 | 60 | 18 | 75 |
| 49 | 11 | 15 | 12 | 19 |
| 42 | 7 | 15 | 8 | 17 |
| 53 | 6 | 95 | 5 | 200 |
| 46 | 3 | 19 | 4 | 26 |
| 33 | 8 | 6 | 16 | 248 |
| 39 | 5 | 76 | 5 | 140 |
| 30 | 96 | 550 | 224 | 1367 |
| 42 | 8 | 340 | 10 | 806 |
| 32 | 215 | 207 | 250 | 235 |
| 36 | 30 | 500 | 203 | 907 |
| 37 | 17 | 91 | 22 | 121 |
| 27 | 54 | 256 | 76 | 353 |
| 33 | 67 | 891 | 72 | 2006 |
| 52 | 40 | 16 | 20 | 859 |
| 41 | 21 | 380 | 27 | 676 |
| 31 | 111 | 296 | 156 | 405 |
| 32 | 14 | 2 | 221 | 574 |
| 34 | 3 | 15 | 3 | 19 |
| 41 | 40 | 84 | 60 | 24 |
| 40 | 96 | 86 | 125 | 97 |
| 50 | 180 | 90 | 21 | 323 |
| 48 | 8 | 14 | 10 | 15 |
| 38 | 50 | 20 | 57 | 41 |
| 36 | 120 | 176 | 11 | 2204 |
| 27 | 10 | 16 | 33 | 256 |
| 35 | 175 | 187 | 202 | 259 |
| 38 | 4 | 23 | 4 | 29 |
| 40 | 101 | 397 | 131 | 508 |
| 25 | 171 | 892 | 187 | 1066 |
| 35 | 59 | 161 | 77 | 248 |
| 26 | 103 | 697 | 156 | 1887 |
| 35 | 10 | 37 | 12 | 50 |
| 14 | 14 | 27 | 5 | 41 |
| 36 | 17 | 139 | 29 | 857 |
| 40 | 136 | 403 | 305 | 958 |
| 38 | 25 | 48 | 26 | 41 |
| 30 | 15 | 215 | 37 | 814 |
| 35 | 5 | 15 | 11 | 35 |
| 28 | 10 | 38 | 13 | 59 |
| 30 | 20 | 191 | 29 | 253 |
| 38 | 54 | 1680 | 237 | 4190 |
| 27 | 58 | 295 | 125 | 95 |
p<0.05 significant to increase of urinary epithelial cell count after 200 mg daily consumption of Vitamin E.
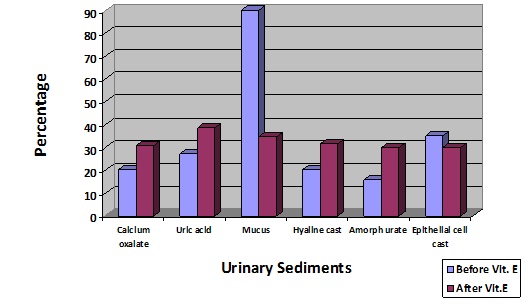
As it evident after administration of vitamin E there was more excretion of urinary death cells in the urine. The effect of vitamin E on urinary sediments i.e. pharmaceutical and epithelial cells casts as well as granular casts in their urine is shown in [Table/Fig-2], vitamin E treatment resulted significantly in the excretion of urinary sediments such as calcium oxalate, uric acid, hydine cast, urates amorph.
Effect of vitamin E on the excretion of urinary sediments of nursing personnel
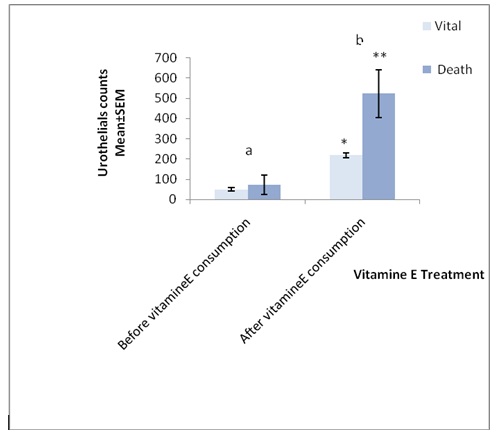
As shown in [Table/Fig-3 and 4], vitamin E significantly effects on urothelial cells counts and the number of vital and death cells were increased from (49.82 ± 8.38 to 73.22 ± 12.65) and (218.93 ±47.22 to 522.8 ± 117.1) respectively (p<0.01).
Effects of vitamin-E on urothelial counts of (n=62) women oncology hospital nurses
p<0.05 significant importance
| Urothelials | Before vitamin E Consumption | 2 week after vitamin E Consumption |
|---|
| n | Mean ± SD | n | Mean ± SD |
|---|
| Vital | n=62 | 49.82 ± 8.38 | n=62 | 73.22 ± 12.65 p=0.018* |
| Death | n=62 | 218.93 ±47.22 | n=62 | 522.8 ± 117.1 p=0.02 |
Effects of vitamin E on urothelial cell counts of (n=62) women oncology hospital nurses
a n=62, t-test, p=0.01
b n=62, t-test, p=0.02
Discussion
In this study, we evaluated urinary sediments, especially uroepithelial tract cells to use them as a main marker; it was appeared that their quantity will change by receiving vitamin E. After receiving vitamin E there were significant increase in the number death urinary epithelial and vital cells among the nursing personnel and also vitamin E caused more excretion of urinary sediments.
In this study, we evaluated urinary sediments, especially uroepithelial tract cells to use them as a main marker; it was appeared that their quantity will change by receiving vitamin E. After receiving vitamin E there were significant increase in the number death urinary epithelial and vital cells among the nursing personnel and also vitamin E caused more excretion of urinary sediments. Vitamin E in both cases before and after receiving has essential roles on epithelial cells counts [13].
Accordingly, Antioxidants, especially vitamin E is the most effective chain-breaking lipophilic antioxidant within biological membranes, can prevent biological damage and has antioxidant property of unsaturated fatty acids of body’s membrane cells and stabilize and preserves membrane of uroepithelial tract cells, and increases their culturing and construction, so these interactions enhance the number of above cells in the urine [14], which is quiet comparable with our results. Hence, since oncology nurses are subjected to chemical therapeutics drugs such as cyclophosphamide etc. which has been shown to have potent carcinogenic activity on cells of urinary tract, and several antineoplastics drugs in job setting, resistance of their body cells may decrease and they may encounter with oxidation stress of cellular components of different types of body cells. Also, the results showed that mucus and urinary casts was used as a second marker in this study.
It seems increases as a result of culturing and construction of urinary tract cells and separation of death epithelial cells, and increased trace value of renal casts and mucoproteins in urinary tracts [15,16].
In summary the count of urinary epithelial cells and urinary sediments in nurses could not be in a fixed range and depends on several factors likes being subjected to job pollutants, antineoplastics drugs, physiological states of a person, age and nutrition.
p<0.05 significant to increase of urinary epithelial cell count after 200 mg daily consumption of Vitamin E.
[1]. Valanis B, Vollmer WM, Labuhn K, Glass A, Corelle C, Antineoplastic drug handling protection afterOSHA guidelines I Occup Med 1992 34:149-55. [Google Scholar]
[2]. Sessink PJ, Boer KA, Scheefhals AP, Anzion RB, Bos RP, Occupational exposure to antineoplastic agents at several departments in a hospital. Environmental contamination and excretion of cyclophosphamide and ifosfamide in urine of exposed workersInt Arch Occup Environ Health 1992 64:105-12. [Google Scholar]
[3]. Auer H, Oehler R, Lindner R, Kowalski H, Sliutz G, Orel L, Characterization of genotoxic properties of difluorodeoxycytidineMutat Res 1997 393:165-73. [Google Scholar]
[4]. Martha E, Laredo B, Carlos A, Lvarez N, Cabiedes J, Urinary sediment analysisReumatol Clin 2010 6:268-72. [Google Scholar]
[5]. Kim Y, Jin DC, Eun Jung Lee EL, Lee DH, Chung HH, Kim M, Lim J, Lee A, Lee K, Kang CS, Pai SH, Quantitative Analysis of Urine Sediment Using Newly Designed Centrifuge TubesAnn Clin Lab Sci 2002 32:55-60. [Google Scholar]
[6]. Graff L, A handbook of routine urinanalysis 1983 PhiladelphiaLippincott [Google Scholar]
[7]. Christian BJ, Loretz LJ, Oberley TD, Catherine A, Reznikoff CA, Characterization of Human Uroepithelial Cells Immortalized in Vitro by Simian Virus 40Cancer Res 1983 47:6066-73. [Google Scholar]
[8]. Zimmermann KC, Bonzon C, Green DR, The machinery of programmed cell deathPharmacol Ther 2001 92:57-70. [Google Scholar]
[9]. Sigounas G, Anagnostu A, Steiner M, Dl-alpha tocopherol induces apoptosis in erythroleukemia, prostate and breast cancer cellsNutr Cancer 1997 28:30-35. [Google Scholar]
[10]. Thamilselvan S, Menon M, Vitamin E, therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status, Vattikuti Urology Institute and Henry Ford Health Sciences Center, Detroit 2005 Michigan, USA [Google Scholar]
[11]. Martha E, Laredo B, Carlos A, Lvarez N, Cabiedes J, Urinary sediment analysisReumatol Clin 2010 6:268-72. [Google Scholar]
[12]. Evstigneeva RP, Volkov IM, Chudinova VV, Vitamin E as a universal antioxidant and stabilizer of biological membranesMembr Cell Biol 1998 12(2):151-72. [Google Scholar]
[13]. Rezei-Basiri M, Rezazadeh H, Aswadi-Kermani I, Eghbal MA, Study on the antimutagenicity effects of Vitamin E in hospital nursing personals and control group usingAmes assay and Comet assay 2012 http://www.irct.ir/user.php [Google Scholar]
[14]. McPherson Henry’s Clinical Diagnosis and Management by Laboratory Methods, Basic examination of urine. Richard A. McPherson, Jonathan Ben-Ezra 2011 22nd edSaundersElsevier:445-79. [Google Scholar]
[15]. Kagan VE, Serbinova EA, Bakalova RA, Stoytchev TS, Erin AN, Prilipko LL, Evstigneeva RP, Mechanisms of stabilization of biomembranes by alpha-tocopherol. The role of the hydrocarbon chain in the inhibition of lipid peroxidationBiochem Pharmacol 1990 40:2403-13. [Google Scholar]
[16]. Villacorta L, Graça-Souza AV, Ricciarelli R, Zingg JM, Azzi A, alfa-Tocopherol Induces Expression of Connective Tissue Growth Factor and Smooth Muscle Cells Antagonizes Tumor Necrosis Factor. Mediated Down regulation in HumanCirc Res 2003 92(1):104-10. [Google Scholar]