Oral and oro-pharyngeal carcinomas are the sixth most common cancers in the world [1]. It is one of the highest occurring cancers where tobacco consumption in any form is common. More than a quarter of the newly diagnosed cancers in males from Sri Lanka, India, Pakistan and Bangladesh are located in the head and neck region [2]. Anterior two third of the tongue is the most common site of the oral cancer, accounting for about 40% of the cases. Most malignancies occur on the lateral border and ventral surface. Despite evolution in management, the overall survival of patients has not improved significantly during the past 20 years, with 5-year survival rates between 45-50% [3]. Several clinico-pathological parameters have been implicated in prognosis, recurrence and survival, of oral squamous cell carcinoma.
In this study, we have tried to find out the prognostic factors for nodal metastasis and recurrence in clinically node negative patients.
To find out the prognostic factors for recurrence and nodal metastasis in early oral tongue cancer.
Material and Methods
The present study was a prospective study. The cases of early cancer of anterior two third of tongue (oral tongue) with ‘clinically N-0’ neck were managed by upfront surgery. Surgeries performed for the primary tumour were ‘wide excision’ with more than 1cm healthy margin all around, or ‘hemiglossectomy’, along with neck dissection. Patients were then given Post-Operative Radiotherapy (PORT) according to standard guidelines.
The inclusion criteria were
Early oral tongue cancer i.e., T1 or T2 primary tumour.
Clinically N-0 neck.
Patients managed by upfront surgery.
The exclusion criteria were
Tumour more than 4cm in greatest diameter.
Tumour crossing midline or reaching midline.
Tumours involving base of tongue or grossly invading floor of mouth.
Those who were given neo-adjuvant chemotherapy.
Clinically palpable cervical lymphnodes.
Carcinoma tongue as second primary in oral cavity.
Span: September 2008 to June 2011.
A total of 63 cases of early oral tongue malignancies were admitted and operated in various units of surgical Oncology Department of The Gujarat Cancer and Research Institute (GCRI) from September 2008 to Aug. 2009. They were analysed using a detailed proforma. All patients were followed till June 2011. Three patients were lost to follow-up, all in first year of surgery, so excluded of study.
Results
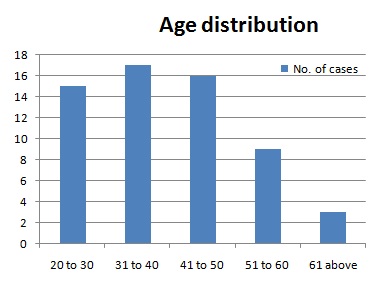
The mean age of presentation was 41.9 years, with range being 22 to 84 years. This study had 50 males and 10 females. 44 out of 60 (73 %) were tobacco chewers. The usual way of presentation is painless ulcer on tongue, with average duration of symptoms being 2.8 months. Only 4 out of 60 patients (6.6%) presented with local pain.
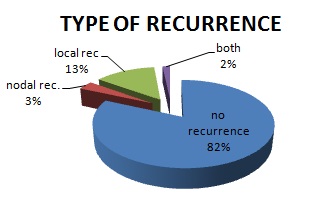
Out of 60 patients, 13 patients underwent Supra-Omohyoid Lymph Node Dissection (SOHND) and rest had Modified Neck Dissection (MND). Recurrence was seen in 11 out of 60 patients (18.3%), in an average follow-up period of about 28 months. Among those who recurred, one patient had both nodal and local recurrence, 2 patients had nodal only (regional) recurrence and rest 8 patients had local recurrence. Out of 13 patients who underwent SOHND, one had nodal recurrence at level 4 (7.69%). The endophytic (ulcero-infiltrative) presentation of disease is more common than exophytic (proliferative) (32 vs. 27). The recurrence rate is also more in endophytic disease 21.8% vs. 14.8% (7vs.4). The average depth of invasion among those who had recurrence was 8.3 mm, in comparison to only 6 mm in those without recurrence p<0.05.
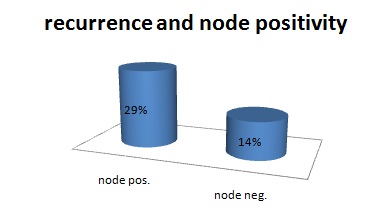
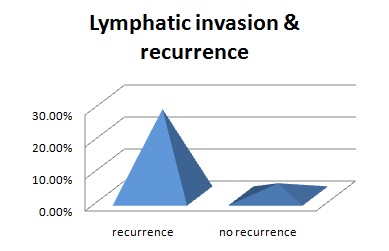
Overall 17 patients out of 60 had pathologically node positive disease; hence, the rate of occult nodal metastasis is 28.3%. Among them none had node positivity at level 5, mostly the nodes were positive for metastasis at level 2 or 3. The recurrence rate among those who were node positive is 29.4 % (5 out of 17), as compared to only 13.9% (6 out of 43). All 3 patients who presented with nodal recurrence had pathologically node positive disease. Lymphatic invasion was seen in 27.2% vs. 4.0% cases in recurrent and non-recurrent group respectively p<0.05. Vascular invasion was seen in only one patient and there was no peri-neural invasion in any patient. The mean healthy surgical margin among the patients with recurrence was 3.1 mm, in comparison to 4.2 mm in the patients without recurrence p<0.05.
Tongue being a muscular organ, muscle invasion is quite common. A total of 51 out of 60 specimens i.e., 85% had muscle invasion. Muscle invasion was seen in 10 out of 11 patients (90.9%) with recurrence and in 41 out of 49 patients (83.6%) without recurrence p<0.05.
In our study, 33 patients had well differentiated and rest 27 had moderately differentiated tumour. None of the patients had poorly differentiated tumour (all poorly differentiated tumours had palpable nodes at presentation, so not included in study). In patients with recurrence, 81.8% of them had well differentiated tumour p> 0.05. A total of 43 out of 60 patients received PORT. 9 out of 11 patients (81.8%) with recurrence received adjuvant radiotherapy. Out of those 43 patients who received PORT, recurrence was seen in 9 (20.9%) [p < 0.05] [Table/Fig-1,2,3,4and5].
Recurrence and node positivity
Lymphatic invasion & recurrence
Parameter and Recurrence rates
| Parameter | Recurrence | No recurrence | Significance |
|---|
| Endophytic disease | 21.8% | 14.8% | p<0.05 |
| Depth of invasion | 8.8mm | 6.1mm | p<0.05 |
| Lymphatic invasion | 27.2% | 4.0% | p<0.05 |
| Muscle invasion | 90.9% | 83.6% | p<0.05 |
| Healthy margin | 3.1mm | 4.2mm | p<0.05 |
| Differentiation Moderate | 81.8% | 48.9% | p>0.05 |
| Adjuvant radiotherapy | 20.9% | 11.76% | p<0.05 |
Discussion
United States (SEER) data reported that the majority of oral cancer patients were over 45 years of age, with a median age of diagnosis at 62 years [4] About 6% of oral cancers occur in young people under the age of 45 years [5]. Oral cancer is known to affect more males than females with an approximate ratio of 1.5:1 [2]. Discrepancy in age distribution and male female ratio (5:1) in our study is due to high prevalence of tobacco chewing habit in the population, more among males than females.
Currently the gold standard treatment is surgery. The aim of surgical ablation for oral cancer is the removal of all viable tumour tissue and detailed pathological examination of specimen that gives risk stratification of disease. This intuitively is associated with better overall prognosis [6]. Radiotherapy has been proposed as another curative modality, or as concurrent or adjuvant with chemotherapy.
Elective neck dissection may be both diagnostic and therapeutic. It helps in defining the status of the neck, removal of undetectable metastasis and determines the need for adjuvant therapy [7]. It is widely accepted that management of neck relapse after a period of observation is far more difficult due to increased incidence of high stage neck disease, along with extra capsular spread. So, some form of treatment to neck should be given even in clinically N-0 cases, in form of either neck dissection or radiotherapy. Elective neck dissection is employed when the risk of cervical involvement is over 15-20% [8].
The risk for cervical node metastases is influenced by characteristics of the primary tumour such as location, size, and histology. Worse prognosis is expected in patients with nodal disease, [9] this worsens with the presence of extra-capsular spread [10]. The incidence of occult lymph node metastasis in early stage tumours (T1/T2) has been reported to be between 27%-40% [11–13]. Such node positive patients should be offered adjuvant radiotherapy, as also stated in NCCN-2011 guidelines. Until recently, depth of invasion has been regarded as the most accurate predictor of metastatic lymphadenopathy. Obviously, the status of ipsilateral neck is important in assessing the risk to the contra-lateral neck. True recurrence develops much earlier than metachronous disease and carries worse prognosis [14].
In a recent study by Jerjes et al., 5 years recurrence rate was 34.9% in early primary tumors of the oral tongue [15].
Several clinico-pathological parameters are being discussed in relation to incidence, recurrence, metastasis, disease progression and survival.
Tumour size
The tumour size usually affects choice and outcome of treatment [16]. Increased tumour size has been linked to cervical nodal involvement, high recurrence rate and poor prognosis [14,17].
Depth of invasion (Tumour thickness)
Depth of invasion is defined as distance between normal mucosal surface and the deepest point of invasion. A precise clinically optimal tumour thickness cut-off point has not been established [7]. In a meta-analysis, sixteen relevant studies were examined for the cut-off tumour thickness points (3,4,5 and 6mm); there was a statistically significant difference between the 4 mm and 5 mm tumour thickness cut-off points and cervical lymph node involvement in OSCC [14]. It is now widely accepted that thickness is more accurate predictor of sub-clinical nodal metastasis, local recurrence and survival than tumour size [14]. The average depth of lesion among those who had recurrence was 8.3 mm, in comparison to only 6 mm in those without recurrence.
TNM staging system
The ‘TNM’ classification of the International Union Against cancer (UICC) relates well to the prognosis and overall survival, earlier the tumor stage, better the prognosis and less complicated is the treatment [18]. Logistic regression analysis revealed that higher the pathological TNM stage, worse the prognosis [15]. There is a growing concern that TNM staging is insufficient to accurately map or classify OSCC, whose biological impact may be related to volume and pathological aggressiveness of disease [14].
Differentiation
The WHO grading system [19] recommends 3 categories: well differentiated, moderately differentiated and poorly differentiated. This usually depends on the subjective pathological assessment of the degree of keratinisation, cellular and nuclear pleomorphism, and mitotic activity [14]. It is a significant predictor of loco-regional failure and tumor recurrence [20]. Multivariate analysis study showed that tumor grade was significantly related to nodal disease at the time of diagnosis. Poorly differentiated tumors usually present with cervical nodal disease [21]. In our study, there was not a single poorly differentiated tumor. So this should be considered as a poor prognostic factor for nodal metastasis. In patients with recurrence, 81.8% of them had well differentiated tumor. This is against the universal finding that recurrence is more common among tumors with higher grades of differentiation [15, 16, 20, 21].
Invasive front (IF)— pattern of invasion
Invasive front (tumour cells at the most invasive part of the malignant tumour) differs significantly from the central or superficial part of the tumour [22]. Understanding the biological behavior of these cells has lead to the link between these cells and the risk of cervical metastasis in OSCC patients [23]. The pattern of invasion can be assessed by using Anneroth et al., and Bryne et al., criteria. Grade 1 tumours had well-delineated “pushing or cohesive” borders. In Grade 2, the advancing edge of tumour infiltrated in solid cords, bands or strands. Grade 3 tumours had margins that contained small groups or cords of infiltrating cells. In Grade 4, there is marked dissociation in small groups or even single cells (non-cohesive) [24]. Image and flow cytometric analysis of the invasive front cells showed abnormal DNA content (4cER), thereby confirming that this can give additional useful information when selecting treatment strategies [25]. A recent study reported that a weak or limited lymphocyte response at the tumour/host interface is strongly associated with local recurrence and death [26].
Endophytic growth pattern is associated with increased local recurrence. High grades of infiltration (grade 3 or 4) are usually associated with nodal involvement and subsequent disease metastasis; while this was not associated with local recurrence. Pattern of invasion didn’t affect cumulative survival.
The endophytic (ulcero-infiltrative) presentation of disease is more common than exophytic (ulcero-proliferative) in our study. The recurrence rate is also more in endophytic disease 21.8% vs. 14.8% (7 vs. 4). So, endophytic growth pattern is associated with increased local recurrence, this was also stated by Spiro RH et al., [24].
Tumour clearance (positive or close surgical margins)
The UK guidelines consider both mucosal and deep margins of 5 mm and more as clear, 1-5 mm as close and less than 1m as involved [27]. This usually ignores the formalin-shrinkage effect which can be at least 30% [24]. So, in order to achieve a 5m pathological clearance, 8-10 mm in situ surgical margin need to be taken [28]. Positive or close margins are associated with increase in local recurrence and have a negative effect on survival [29,30]. In our study, the mean minimal surgical margin among the patients with recurrence is 3.1mm, in comparison to 4.2m in the patients without recurrence. Most of the patients had close margin at base of resection or towards base of tongue. So, such cases should be advised post-operative radiotherapy.
Presence of Severe Dysplasia (SD) or dysplasia at margin
The presence of mild or moderate epithelial dysplasia at the margins of surgically removed OSCC carries a significant risk for the development of local recurrence [31]. Dysplasia at margin is an excellent predictor of tumour spread.
Lympho-vascular and nerve invasion
Lympho-vascular and peri-/endoneural invasion show a significant association with tumour size, histological grading, invasive front, nodal involvement, status of the surgical margins, overall prognosis and survival [16]. It has been proposed that tumour emboli are more difficult to form in the small-caliber lymphatics of superficial areas than in the wider lymphatics of deep tissue, hence tumour thickness may play a vital role in lympho-vascular invasion [32,33].
In our study, lymphatic invasion is seen in 27.2% vs. 4.0% in recurrent and non-recurrent group respectively. So, it can be inferred that patients with lymphatic invasion should be treated with adjuvant radiotherapy.
Radiotherapy
Radiotherapy plays a key role in the management of oral cavity SCC, either alone or more frequently combined with surgery and/or chemotherapy [34]. The use of very accurate surgically directed radiotherapy in the form of brachytherapy for very early disease may be justified in some cases i.e., where the surgical sequelae outweigh the disadvantage of using a modality which in essence can only be used once. We must consider carefully the bystander tissue irradiation which may have a significant adverse host effect when treating localized early cancer [35].
Conclusion
Proposed new clinico-pathological GCRI-classification of early tongue (or oral cavity) cancers that may guide towards therapy is,
The disease should be classified according to aggressiveness, not merely on the basis of size (T) and lymph node metastasis (N).
1. Type I – Non aggressive: Superficial tumours with pushing type margins, no lympho-vascular or perineural invasion and pathologically node negative.
2. Type II – Aggressive disease: Lesions with any one of the following adverse features:
Node positive disease.
Infiltrative lesions with depth more than 6mm or.
Lympho-vascular invasion.
Close surgical margins.
Lesions showing dysplasia at margins.
Poorly differentiated lesion.
3. Type III – Highly aggressive: Node positive disease with extra capsular spread or R-1 or R-2 resection.
4. Type IV – Metastatic (M-1).
Surgery alone with good healthy margin is sufficient for Type I tumours. Surgery followed by post-operative radiotherapy should be used for Type II (aggressive tumours), and combined modality therapy should be used for Type III tumours including surgery & adjuvant chemo-radiation. In node negative patients, selective node dissection including level 1, 2, 3 and 4, is a viable option for neck to decrease the morbidity of MND. Type 4 disease patients should be given best palliation.