Introduction: Coronary heart disease is one of the most common cardiac health problem in India. Anti-platelet therapy is the cornerstone in the management of coronary heart disease. The current study was undertaken to compare the effect of different oral anti-platelet regimens on percentage inhibition of platelet aggregation in coronary heart disease patients using chronolog light transmittance aggregometry.
Material and Methods: Blood samples of 215 consecutive patients diagnosed of coronary heart disease (Male: Female ratio- 142: 73) with mean age of 55.2 ±10.3 years, who underwent platelet aggregation test were analysed. Patients were either on aspirin, clopidogrel, prasugrel, cilostazol or a combination of these drugs in different dosages. Of the 215 coronary heart disease patients, 35, 115 and 65 patients were on single, dual and triple anti-platelet drug regimen respectively.
Results: The Percentage Inhibition of Platelet Aggregation (%IPA) in patients on dual anti-platelet regimen was highest i.e., 65.14 ± 23.23 as compared to 48.89 ± 22.16 in patients on monotherapy and 62.14 ± 21.64 in patients on triple anti-platelet regimen. Percentage of responders (> 40% inhibition of platelet aggregation) were 54.28%, 73.91% and 64.61% in single, dual and triple drug regimens respectively. Among responders on dual anti-platelet regimen, 64.7% were on aspirin + prasugrel and 35.3% were on aspirin + clopidogrel. The Percentage Inhibition of platelet aggregation in diabetics on dual anti-platelet regimen was 71.69 ± 17.54 as compared to 56.14 ± 23.29 in diabetics on triple anti-platelet regimen.
Conclusion: Dual anti-platelet therapy containing prasugrel was found to be more effective than dual anti-platelet therapy containing clopidogrel on background aspirin therapy and triple anti-platelet therapy in terms of percentage inhibition of platelet aggregation in coronary heart disease patients especially those with concomitant diabetes, however this conclusion needs to be further confirmed by large-scale randomized clinical trials.
Coronary Heart Disease (CHD), Oral Anti-platelet therapy, Light Transmittance Aggregometry (LTA), Dual anti-platelet regimen, Triple anti-platelet regimen, Percentage Inhibition of Platelet Aggregation (% IPA)
Introduction
Coronary Heart Disease (CHD) is most predominant among the cardiovascular diseases and ranked number one in prevalence among the developing countries [1]. Coronary heart disease is an epidemic in India and one of the major causes of disease-burden and deaths [2]. Platelets have an established role in the pathogenesis of atherosclerosis-related coronary heart diseases [3]. Platelet adhesion, activation, and aggregation play an integral role in the development of platelet rich thrombus and ischaemic complications in coronary heart disease [4]. Oral anti-platelet drugs are cornerstone of modern pharmacotherapy in cardiovascular atherothrombotic diseases [5]. Current oral anti-platelet agents target the TxA2 (aspirin) and ADP (P2Y12 inhibitors, such as clopidogrel, ticlopidine, and prasugrel) platelet activation pathways and have been demonstrated to significantly reduce the incidence of ischaemic events in patients with atherothrombotic disease [6]. The well-documented efficacy of aspirin and clopidogrel has been recognized by the American College of Cardiology/American Heart Association guidelines, [7] and clinical trials also have shown that combining clopidogrel with aspirin resulted in an additional 20% reduction in nonfatal myocardial infarction, stroke, and death compared with aspirin alone [8]. Triple-anti-platelet therapy with cilostazol, aspirin, and clopidogrel reduced long-term cardiac and cerebral events in patients with high ACS risk profile [9]. Despite the established benefits of aspirin and ADP receptor inhibitors, these agents are associated with important clinical limitations, including a high residual risk for ischaemic events, elevated bleeding risk, and variable inhibition of platelet aggregation [6]. Hence, monitoring of anti-platelet therapy is essential due to variability in anti-platelet response seen with aspirin and ADP receptor inhibitors. An ideal platelet function test would be rapid, simple and reproducible. Among various methods available, turbidometric Light Transmittance Aggregometry (LTA) is still regarded as the gold standard of platelet function testing. LTA measures platelet aggregation in platelet-rich plasma following in vitro stimulation with various agonists and is the most widely investigated method to predict clinical outcome [10]. This method monitors anti-platelet effects of aspirin, clopidogrel and GP IIb/IIIa antagonists. Resistance to oral anti-platelet drugs may be attributed to variable inhibition of platelet aggregation and their correlation to the clinical outcome and adverse events [11]. In addition, Gum et al., defined aspirin resistance as >70 % aggregation with 10 μm ADP on LTA despite regular intake of aspirin [12]. Clopidogrel resistance is defined as inhibition of platelet aggregation < 30% with 10 μM ADP using LTA [13]. Platelet function response evaluation by light transmission aggregometry based on percentage inhibition of platelet aggregation (% IPA) is more than 40%, 30-40% and less than 30% for responders, poor responders and non responders respectively for both aspirin and clopidogrel [14]. Consequently, ADP induced platelet aggregation with this threshold (>70%) appears relevant not only to isolate the low responders to clopidogrel, but also more largely to the dual anti-platelet therapy. Dual resistance to both aspirin and clopidogrel may contribute to recurrent events like myocardial infarction, stroke or death [14]. The present study was thus designed to compare the effect of different anti-platelet regimens on platelet inhibition using platelet aggregometry test i.e., (Chronolog Light Transmittance Aggregometry) in coronary heart disease patients.
Material & Methods
Study population
The present observational study was done in the Department of Clinical Pharmacology & Therapeutics at Nizam’s Institute of Medical Sciences. The study protocol was approved by NIMS Institutional Ethics Committee. At the time of sample collection, written informed consent was taken from the patient and the patient related data, medical history, diagnosis, laboratory values and given treatment were noted in a case record form. All consecutive coronary heart disease patients who came to the department for platelet aggregation test were included in the study. Patients with a history of bleeding diathesis, contra-indications to anti-platelet therapy and platelet count less than one lakh/mm3 were not included in the study. A total of 215 samples of patients diagnosed with coronary heart disease were monitored from January 2013 to May 2013 for platelet inhibition using Chronolog light transmittance aggregometry.
Measurement of platelet aggregation
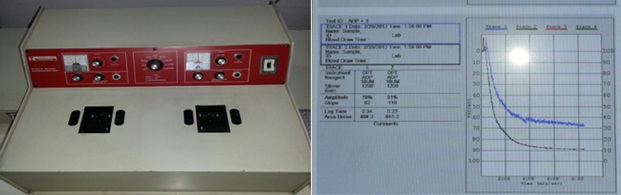
Measurement of platelet aggregation was done by using a dual-channel platelet aggregometer (Chrono-Log 490 Model, Chrono-Log Corp, Havertown, PA, USA as shown in [Table/Fig-1] by the turbidimetric method. Nine ml of blood was collected from the antecubital vein in a polypropylene test tube containing 1ml of sodium citrate. Platelet Rich Plasma (PRP) was obtained as a supernatant after centrifugation of citrated blood at 800 rpm for 15 minutes. The isolated PRP was kept at 37°C before use in a polycarbonate cuvette. Platelet-poor plasma (PPP) was obtained by a second centrifugation of the blood fraction at 2500 rpm for 10 minutes [15]. Platelet aggregation was assessed with 0.5 ml of Platelet-Rich Plasma (PRP) after a stable baseline had been established by using Adenosine Diphosphate (ADP) 10 μmol/ml as agonist in cuvettes containing stir bars. This is a fixed wavelength spectrophotometer with two sample chambers heated to 37°C, containing a magnetic stirrer which mimics the shear conditions during blood flow. The principle of LTA is low shear platelet to platelet aggregation in response to classical agonists [16]. The transmission was set to zero on the chart recorder. The transmission of infra red light through two cuvettes, one containing PRP as sample and one containing Platelet Poor Plasma (PPP) from the same subject as reference, was measured at baseline and until 6 minutes after the administration of the agonist. Signals were transferred to a computer with Aggrolink software (Chrono-Log Corporation, Haverton, PA) and the transmission data obtained until 6 minutes after agonist administration were used to calculate maximum platelet aggregation [17]. Then, results were calculated by the software and the amplitude of the sample tracing gives the value of platelet aggregation in percentage (% PA). The percentage inhibition of platelet aggregation (% IPA) was then calculated by subtracting % PA value from 100. A sample tracing of platelet aggregation using ADP as agonist was shown in [Table/Fig-1].
Dual chamber Chronolog Platelet Aggregometer showing a Sample tracing of platelet aggregation by using 10 μmol/ml ADP
Anti-platelet therapies
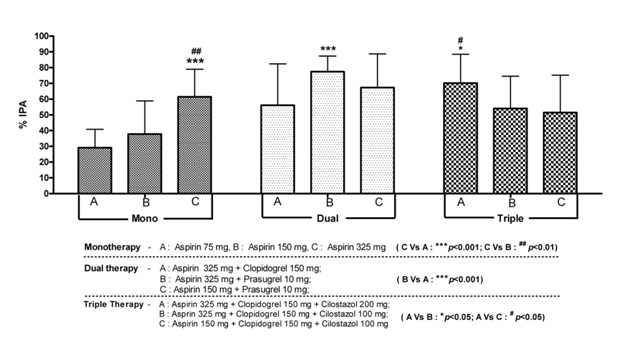
Based on the number of anti-platelet drugs the patients are receiving, they are classified into monotherapy, dual therapy and triple anti-platelet therapy. All CHD patients on monotherapy were either on aspirin 75mg or 150mg or 325mg. Coronary heart disease patients on dual therapy were either on aspirin 325mg + clopidogrel 150mg or aspirin 325mg + prasugrel 10mg or aspirin 150mg + prasugrel 10mg. Coronary heart disease patients on triple therapy were either on aspirin 325mg + clopidogrel 150mg + cilostazol 200mg or aspirin 325mg + clopidogrel 150mg + cilostazol 100mg or aspirin 150mg + clopidogrel 150mg + cilostazol 100mg.
Statistical Analysis
The data was presented as mean ± SD. Two tailed unpaired t-test was used to compare differences between two groups. One way ANOVA was used to compare the difference within and between the groups. The level of significance was set at p<0.05 with a power of 90%. The statistical analysis was performed using the Graph pad PRISM software 4 (Graph pad software Inc., USA).
Results
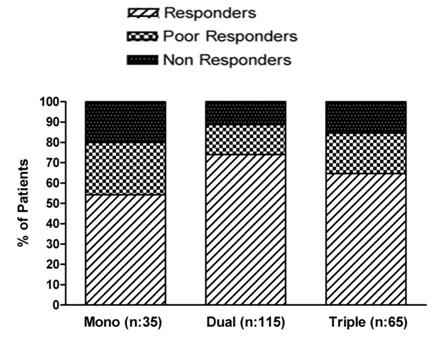
A total of 215 coronary heart disease patients were included in the study and baseline demographic data is shown in [Table/Fig-2]. Out of 215 patients, 35 were on single anti-platelet therapy, 115 were on dual anti-platelet therapy and 65 on triple anti-platelet therapy. Among them, responders are 19 in single, 85 in dual and 42 in triple anti-platelet therapy. The percentage of responders (as defined by > 40% IPA) in single, dual & triple anti-platelet therapy are 54.28%, 73.91% and 64.61% respectively. The percentage of poor responders (as defined by % IPA in the range of 30 to 40) in single, dual & triple anti-platelet therapy are 25.71%, 14.78% and 20% respectively. The percentage of non responders (as defined by % IPA less than 30) in single, dual & triple anti-platelet therapy are 20%, 11.30% and 15.38% respectively (as shown in [Table/Fig-3].
Demographic characteristics
| Single anti-platelet therapy | Dual anti-platelet therapy | Triple anti-platelet therapy |
|---|
| Age in years (mean±SD) | 55.18 ± 12.3 | 54.55 ± 11.2 | 56.14 ± 9.8 |
| Gender (Male: Female) | 28:7 | 76:39 | 38:27 |
Percentage of Response to different anti-platelet regimens
The % IPA in patients on dual anti-platelet regimen was highest i.e., 65.14 ± 23.23 as compared to 48.89 ± 22.16 in patients on monotherapy and 62.14 ± 21.64 in patients on triple anti-platelet regimen (p < 0.001 compared to monotherapy & p= ns compared to triple anti-platelet therapy) as shown in [Table/Fig-4]. Further analysis of different anti-platelet regimens revealed that among patients on monotherapy with aspirin, there is a dose dependent increase in the % IPA (29.14 ± 11.74, 37.78 ± 21.03 and 61.42 ± 17.49 with 75, 150 and 325mg doses of aspirin respectively) which is statistically significant (p value < 0.01). Among responders in dual anti-platelet regimen, 64.7% were on aspirin + prasugrel whereas 35.3% were on aspirin + clopidogrel. The % IPA in patients on dual anti-platelet therapy was higher with asprin 325mg + prasugrel 10mg regimen as compared to aspirin 325mg + clopidogrel 150mg regimen (77.43 ± 9.89 Vs 56.07 ± 26.26 with a p value < 0.001). Among 42 responders in triple anti-platelet regimen, 22 were given aspirin 325mg + clopidogrel 150mg + cilostazol 200mg, 10 were given aspirin 325mg + clopidogrel 150mg + cilostazol 100mg, another 10 were on aspirin 150mg + clopidogrel 150mg + cilostazol 200mg and none of them were given prasugrel as a part of triple anti-platelet regimen. The % IPA in patients on triple anti-platelet therapy was higher with asprin 325mg + clopidogrel 150mg + cilostazol 200mg regimen as compared to aspirin 325mg + clopidogrel 150mg + cilostazol 100mg regimen and aspirin 150mg + clopidogrel 150mg + cilostazol 200mg regimen (70.17 ± 18.27 Vs 54.07 ± 20.43 Vs 51.47 ± 23.66 with a p value < 0.05) as shown in [Table/Fig-5].
Comparison of percentage IPA with Mono, Dual and Triple Regimens
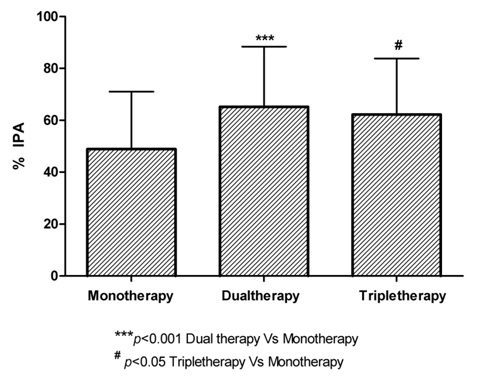
Percentage IPA in patients on Mono, Dual and Triple Anti-platelet Regimens
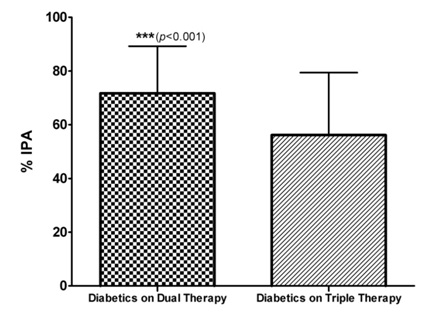
Among 215 coronary heart disease patients, 90 were diabetics and 125 were non-diabetics. Out of 90 Diabetic CHD patients, 61% were prescribed prasugrel as a part of dual anti-platelet regimen along with aspirin whereas 11% were prescribed clopidogrel as a part of dual anti-platelet regimen along with aspirin and 28% were on triple anti-platelet regimen. The % IPA in diabetics on dual anti-platelet regimen was 71.69 ± 17.54 as compared to 56.14 ± 23.29 in diabetics on triple anti-platelet regimen (p value <0.001) as shown in [Table/Fig-6]. Among diabetics on dual anti-platelet regimen, % IPA with aspirin 325mg + clopidogrel 150mg was 57.53 ± 18.33 and % IPA with aspirin 325mg + prasugrel 10mg was 75.03 ± 15.26.
Comparison of percentage IPA in Diabetics on Dual and Triple anti-platelet Therapies
Discussion
The present study was undertaken to evaluate and compare the effect of different oral anti-platelet drug regimens in coronary heart disease patients by using light transmittance platelet aggregometry test. The goal of oral anti-platelet therapy is to provide maximal protection against thrombosis without increasing the risk of bleeding [18]. Anti-platelet agents have been used in acute conditions of coronary artery thrombosis and as part of secondary prophylaxis to prevent recurrent thromboembolic episodes [19]. Inspite of taking oral anti-platelet drugs in appropriate doses, recurrent episodes of acute coronary events are still common [20]. This might be due to the presence of co-morbid conditions like diabetes mellitus, hypertension and hyperlipidemia or could be due to anti-platelet resistance. Coronary heart disease patients with T2DM have increased platelet reactivity and reduced in vitro responsiveness to anti-platelet agents, which include P2Y12 receptor antagonists, compared with nondiabetic subjects [21]. Data have linked therapeutic failure (resistance, low-or hypo-responsiveness) to anti-platelet therapy to an increased risk of cardiovascular complications including stent thrombosis [22]. Compared to clopidogrel resistance, ASA resistance has so far received little attention in interventional cardiology. A special group to be considered is patients with dual anti-platelet resistance as these patients bear the greatest risk of major adverse events, such as stent thrombosis [23]. An analysis of the RECLOSE trial cohort showed a prevalence of 6% with dual resistance to aspirin and clopidogrel [14]. Other data revealed a prevalence of 10.4% dual low response and suggest a high cardiovascular risk after PCI for these patients with the need for intensified anti-platelet therapy and follow-up [24]. Our study results reveal that among patients on dual anti-platelet regimen with aspirin and clopidogrel, the percentage of poor responders and non responders were 7.9% and 6.04% respectively. Niitsu et al., [25] showed that dual anti-platelet therapy with aspirin and prasugrel produced better anti-platelet activity than either agent used alone. Prasugrel is approximately 10- and 100-fold more potent in inhibiting platelet function in vivo than clopidogrel and ticlopidine, respectively [26]. In addition, the pharmacologic non responders were fewer with prasugrel compared with clopidogrel (3% vs 52%, respectively) [27]. Similarly, our study results show that the % IPA in patients on dual anti-platelet therapy was higher with aspirin 325mg + prasugrel 10mg regimen as compared to aspirin 325mg + clopidogrel 150mg regimen (77.43 ± 9.89 Vs 56.07 ± 26.26 with a p-value <0.001) and there were no pharmacologic non responders in aspirin + prasugrel regimen. Clinical trials have proven that prasugrel produces faster anti-platelet activity and lesser interpatient variability when compared with clopidogrel. In light of this, compared with clopidogrel, prasugrel is suggested to have a faster onset of action, greater potency, and lesser individual variability in inhibition of platelet function [28]. The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI 38 study) [29] compared the use of prasugrel with clopidogrel in patients on standard aspirin background therapy with moderate to high risk of ACS. Patients with diabetes mellitus had greater benefit compared with nondiabetic patients when dual anti-platelet therapy with prasugrel was used. In a sub-analysis of the TRITON-TIMI 38 study, prasugrel use was more efficacious compared with clopidogrel in reducing the ischaemic event rates in both diabetics and nondiabetics, with greater reduction in diabetics [30]. This suggests that the greater anti-platelet activity produced by prasugrel resulted in higher net clinical benefit in patients with DM when compared with patients without DM. The results of the Optimizing Anti-platelet Therapy in Diabetes Mellitus (OPTIMUS-1) study demonstrated that a high-clopidogrel maintenance dose was associated with greater platelet inhibitory effects compared with standard dosing in patients with DM and CAD who presented with elevated platelet reactivity while on standard dual anti-platelet therapy [15]. Similarly, our study results show that high dose clopidogrel maintenance dose as a component of dual and triple anti-platelet regimens in patients with coronary heart disease and concomitant diabetes has shown % IPA of 56.07 ± 26.26 and 58.57 ± 20.33 respectively. The OPTIMUS-2 study showed that adjunctive treatment with cilostazol (‘triple therapy’) enhances measures of platelet P2Y12 inhibition in patients with DM and CAD to a greater extent than standard dual anti-platelet therapy with aspirin and clopidogrel [31]. In contrast, our study results have shown better platelet inhibition with dual anti-platelet therapy when compared to triple anti-platelet regimen (% IPA of 71.69 ± 17.54 Vs 56.14 ± 23.29). This change can be explained by the preferential presence of prasugrel in most of the dual anti-platelet regimens rather than clopidogrel. The results of the optimizing anti-platelet therapy in diabetes mellitus (OPTIMUS)-3 Trial has concluded that in patients with type 2 DM and CAD, standard-dose prasugrel is associated with greater platelet inhibition and better response profiles during both the loading and maintenance periods when compared with double-dose clopidogrel [32]. In the PRINCIPLE-TIMI 44 Trial, the rate of response to prasugrel 60 mg loading dose /10 mg maintenance dose was significantly greater than double-dose clopidogrel in the overall patient population, as well as in patients with DM [33]. Similarly, our study results are similar to OPTIMUS 3 study and PRINCIPLE- TIMI 44 Trial in that among diabetics on dual anti-platelet regimen, % IPA with aspirin 325mg + prasugrel 10mg regimen was significantly greater than aspirin 325mg + clopidogrel 150mg regimen (75.03 ± 15.26 Vs 57.53 ± 18.33). Numerous studies as discussed above have shown that prasugrel was found to be effective anti-platelet drug in coronary heart disease patients with concomitant diabetes.
Conclusion
In the present study, dual anti-platelet therapy was found to be superior to triple anti-platelet therapy in terms of percentage inhibition of platelet aggregation. Dual anti-platelet therapy containing prasugrel was found to be more effective as compared to clopidogrel in the background of aspirin therapy in coronary heart disease patients with concomitant diabetes; however this conclusion needs to be further confirmed by large-scale randomized clinical trials.
Study Limitations
Long term follow up with regard to clinical outcome of coronary heart disease patients on different oral anti-platelet regimens with subsequent platelet aggregation tests would be beneficial.
Fewer coronary heart disease patients in triple as compared to dual anti-platelet regimen is another study limitation.
Future Scope
Similar studies will provide a better understanding and highlight the importance of platelet function tests in determining the optimized anti-platelet therapy. Early identification of poor and non-responders by monitoring of anti-platelet effect and initiation of appropriate anti-platelet therapy in right doses is the key to the success of oral anti-platelet regimens in coronary heart disease patients. Therefore, it is likely that the future of anti-thrombotic pharmacology will rely on platelet function tests in order to set the basis for individualized oral anti-platelet treatment regimens.
[1]. Pranavchand R, Reddy BM, Current status of understanding of the genetic etiology of coronary heart diseaseJ Postgrad Med 2013 59:30-41. [Google Scholar]
[2]. Gupta R, recent trends in coronary heart disease epidemiology in IndiaIndian Heart J 2008 60(2suppl-B):B4-18. [Google Scholar]
[3]. Marczewski MM, Postula M, Kosior D, Novel anti-platelet agents in the prevention of cardiovascular complications—focus on ticagrelorVascular health and risk management 2010 6:419-29. [Google Scholar]
[4]. John J, Koshy Santhosh KG, Current oral anti-platelets: focus update on prasugrelJournal of the American Board of Family Medicine: JABFM 2012 25(3):343-9. [Google Scholar]
[5]. Tan Jack WC, Guo Kenneth WQ, Management of anti-platelet therapy during acute percutaneous coronary intervention: new strategies and therapeuticsAnnals of the Academy of Medicine, Singapore 2010 39(3):221-9. [Google Scholar]
[6]. Fintel Dan J, Oral anti-platelet therapy for atherothrombotic disease: overview of current and emerging treatment optionsVascular health and risk management 2012 8:77-89. [Google Scholar]
[7]. Smith SC Jr, Allen J, Blair SN, AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood InstituteCirculation 2006 113(19):2363-72. [Google Scholar]
[8]. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med 2001 345(7):494-502. [Google Scholar]
[9]. Han Y, Li Y, Wang S, Jing Q, Wang Z, Wang D, Cilostazol in addition to aspirin and clopidogrel improves long-term outcomes after percutaneous coronary intervention in patients with acute coronary syndromes: a randomized, controlled studyAmerican heart journal 2009 157(4):733-9. [Google Scholar]
[10]. Sambu N, Curzen N, Monitoring the effectiveness of anti-platelet therapy: opportunities and limitationsBritish journal of clinical pharmacology 2011 72(4):683-96. [Google Scholar]
[11]. Kuliczkowski W, Witkowski A, Polonski L, Watala C, Filipiak K, Budaj A, Interindividual variability in the response to oral anti-platelet drugs: a position paper of the Working Group on anti-platelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the WorkingEuropean heart journal 2009 30(4):426-35. [Google Scholar]
[12]. Gum PA, Kottke-Marchant K, Welsh PA, White J, Topol EJ, A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular diseaseJ Am Coll Cardiol 2003 41:961-5. [Google Scholar]
[13]. Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM, Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pre-treatment platelet reactivityCirculation 2003 107:2908-13. [Google Scholar]
[14]. Gori AM, Marcucci R, Migliorini A, Valenti R, Moschi G, Paniccia R, Incidence and clinical impact of dual non-responsiveness to aspirin and clopidogrel in patients with drug-eluting stentsJ Am Coll Cardiol 2008 52:734-39. [Google Scholar]
[15]. Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Anti-platelet Therapy in Diabetes Mellitus (OPTIMUS) studyCirculation 2007 115(6):708-16. [Google Scholar]
[16]. Harrison P, Frelinger AL, Furman MI, Michelson AD, Measuring anti-platelet drug effects in the laboratoryThrombosis research 2007 120(3):323-36. [Google Scholar]
[17]. Ungerer M, Rosport K, Bultmann A, Piechatzek R, Uhland K, Schlieper P, Novel anti-platelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general haemostasis in humansCirculation 2011 123(17):1891-99. [Google Scholar]
[18]. Bode C, Huber K, Anti-platelet therapy in percutaneous coronary interventionEuropean Heart Journal Supplements 2008 10(Suppl A):A13-A20. [Google Scholar]
[19]. Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, Primary and secondary prevention of cardiovascular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice GuidelinesChest 2012 141(2 Suppl):e637S-68S. [Google Scholar]
[20]. Angiolillo DJ, Anti-platelet therapy in diabetes: efficacy and limitations of current treatment strategies and future directionsDiabetes Care 2009 32:531-540. [Google Scholar]
[21]. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C, Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatmentDiabetes 2005 54:2430-5. [Google Scholar]
[22]. Sofi F, Marcucci R, Gori AM, Giusti B, Abbate R, Gensini GF, Clopidogrel non-responsiveness and risk of cardiovascular morbidity. An updated meta-analysisThromb Haemost 2010 103:841-48. [Google Scholar]
[23]. Neubauer H, Kaiser AFC, Endres HG, Kruger JC, Engelhardt A, Lask S, Tailored anti-platelet therapy can overcome clopidogrel and aspirin resistance—the BOchum CLopidogrel and Aspirin Plan (BOCLA-Plan) to improve anti-platelet therapyBMC medicine 2011 9(1):3 [Google Scholar]
[24]. Ivandic BT, Sausemuth M, Ibrahim H, Giannitsis E, Gawaz M, Katus HA, Dual anti-platelet drug resistance is a risk factor for cardiovascular events after percutaneous coronary interventionClin Chem 2009 55:1171-1176. [Google Scholar]
[25]. Niitsu Y, Jakubowski JA, Sugidachi A, Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent anti-platelet agent with in vivo P2Y12 receptor antagonist activitySemin Thromb Hemost 2005 31:184-94. [Google Scholar]
[26]. Sugidachi A, Ogawa T, Kurihara A, The greater in vivo anti-platelet effects of prasugrel compared to clopidogrel reflect more efficient generation of its active metabolite with similar anti-platelet activity to clopidogrel’s active metaboliteJ Thromb Haemost 2007 5:1545-1551. [Google Scholar]
[27]. Jernberg T, Payne CD, Winters KJ, Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery diseaseEur Heart J 2006 27(10):1166-73. [Google Scholar]
[28]. Jakubowski JA, Winters KJ, Naganuma H, Wallentin L, Prasugrel: a novel thienopyridine anti-platelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct anti-platelet profileCardiovasc Drug Rev 2007 25(4):357-74. [Google Scholar]
[29]. Wiviott SD, Braunwald E, McCabe CH, Prasugrel versus clopidogrel in patients with acute coronary syndromesN Engl J Med 2007 357(20):2001-15. [Google Scholar]
[30]. Wiviott SD, Braunwald E, Angiolillo DJ, Greater clinical benefit of more intensive oral anti-platelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel–Thrombolysis in Myocardial Infarction 38Circulation 2008 118(16):1626-36. [Google Scholar]
[31]. Angiolillo D J, Capranzano P, Goto S, Aslam M, Desai B, Charlton RK, A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual anti-platelet therapy: results of the OPTIMUS-2 studyEuropean heart journal 2008 29(18):2202-11. [Google Scholar]
[32]. Angiolillo DJ, Badimon JJ, Saucedo JF, Frelinger AL, Michelson AD, Jakubowski JA, A pharmacodynamic comparison of prasugrel vs. high-dose clopidogrel in patients with type 2 diabetes mellitus and coronary artery disease: results of the Optimizing anti-Platelet Therapy In diabetes MellitUS (OPTIMUS)-3 TrialEuropean heart journal 2011 32(7):838-46. [Google Scholar]
[33]. Wilson SR, Antman EM, Frelinger AL, O’Donoghue M, Neumann FJ, Michelson AD, Assessment of platelet function in diabetes mellitus: observations from the PRINCIPLE-TIMI 44 trialCirculation 2009 120:S548-S549. [Google Scholar]