A Study of Package Inserts in Southern India
Deepak Ramdas1, Ananya Chakraborty2, Swaroop HS3, Syed Faizan4, Praveen Kumar V5, Srinivas BN6
1 Post Graduate Student, Department of Pharmacology, Vydehi Institute of Medical Sciences and Research CentreWhitefield, Bangalore, India.
2 Associate Professor, Department of Pharmacology, Vydehi Institute of Medical Sciences and Research CentreWhitefield, Bangalore, India.
3 Post Graduate Student, Department of Pharmacology, Vydehi Institute of Medical Sciences and Research CentreWhitefield, Bangalore, India.
4 Undergraduate Student, Second Year, Vydehi Institute of Medical Sciences and Research CentreWhitefield, Bangalore, India.
5 Undergraduate Student, Second Year, Vydehi Institute of Medical Sciences and Research CentreWhitefield, Bangalore, India.
6 Professor and HOD, Department of Pharmacology, Vydehi Institute of Medical Sciences and Research CentreWhitefield, Bangalore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ananya Chakraborty, Department of Pharmacology, Vydehi Institute of Medical Sciences and Research Centre Whitefield, Bangalore-37, India. Email: dr_ananya@yahoo.com
Introduction: Package insert is an officially approved document that accompanies a drug. It is intended to provide information for the safe and effective use of a drug and contains information based on regulatory guidelines. Sometimes, information provided in the package inserts is suboptimal which can led to medication errors. This study was undertaken to assess the presentation and completeness of clinical information provided in the currently available package inserts for anti-diabetic, anti-hypertensive and hypolipedemic drugs in India.
Material and Methods: Around 130 package inserts were collected from pharmacies located at different areas of Bangalore. They were analyzed based on criteria mentioned in Schedule D of Drug and Cosmetic act 1945.
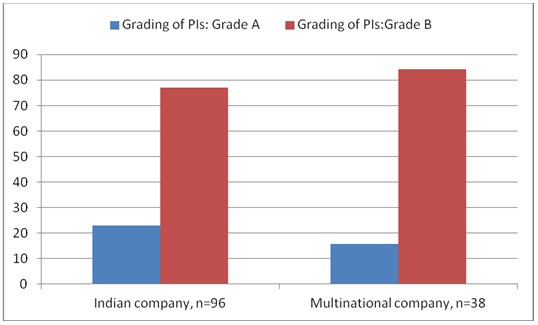
Results and Observations: Out of 134 package inserts, 64 were anti-diabetics, 40 anti-hypertensives, and 30 hypolipedemics. Out of them, 31 (23.14%) belonged to Grade ‘A’ (including all injectable preparations) and remaining 76.86% to Grade ‘B’. None of the PIs belonged to Grade ‘C’. The inserts were inadequate in many aspects; for example, they had unclear instructions about generic name of other ingredients used, about handling, undesirable effects, pediatric and geriatric use, and guidelines for use of the drugs.
Conclusion: This study indicated that information relevant to the safe and effective use of medication was not mentioned in the analyzed package inserts. It is, therefore, recommended to update the existing package inserts based on criteria mentioned in the Schedule D of Drug and Cosmetic Act, 1945.
Drug-information, Package inserts, Anti-diabetic, Anti-hypertensives, Hypolipedemics
Introduction
Accurate and reliable drug information is essential for safe and effective use of marketed products. The primary source of drug information is a Package Insert (PI). It is a printed leaflet that contains information based on regulatory guidelines for the safe and effective use of a drug. It is also known as prescription drug label or prescribing information. A good PI contains the approved, essential, and accurate information about a drug. It is written in a language that is not promotional, false, or misleading. It is evidence-based and is updated time to time as relevant pre-clinical and clinical data becomes available [1]. This drug product information commences early during the developmental phase of a pharmaceutical product with careful scrutiny of available information [2]. In India, the concept of package insert is governed by the ‘Drugs and Cosmetics Act (1940) and Rules (1945). The section 6 of Schedule D (II) of the rules lists the headings according to which information should be provided in the PIs. The ‘Section 6.2’ mandates that the PIs must be in ‘English’ and provides information regarding the specific requirements. The ‘Section 6.3’ mandates pharmaceutical information on list of excipients [3].
Numerous studies have shown that one of the key components in the management of health conditions is the use of prescribed drugs. Unfortunately, patients with chronic conditions like diabetes, hypertension, and dyslipidemia adhere only to 50-60% of prescribed medications [4]. Diabetes mellitus has emerged as a major health care burden with a current prevalence of 8.3% in urban India [5]. According to reports, India will have the largest number of diabetic patients by 2030 [6]. Hypertension is a very common disorder of past middle age with a prevalence of 19.04% in central India. It is an important risk factor for cardio-vascular mortality and morbidity [7]. Hyperlipedimia is one of the important risk factor for diabetes mellitus, hypertension, and coronary artery disease with a prevalence of 30.3% in urban India [8]. Various studies have concluded that PIs because of their easy availability can produce an important impact on patients compliance and thus on the ultimate effectiveness of drug use [9,10]. They also can serve as reliable and accurate sources of drug information for health professionals [11].
Keeping this in mind, this study was designed to assess the presentation and completeness of clinical information provided in the currently available package inserts for anti-diabetic, anti-hypertensive and hypolipedemic drugs in India.
Material and Methods
Collection of PIs: PIs were collected from various pharmacies located in various parts of Bangalore on request over a period of four weeks in the month of March 2013.
Analysis of content of PIs: PIs were scored based on criteria laid down by Indian Drug and Cosmetic Rules, 1945 under section 6.2 of schedule D. Data were extracted twice to minimize chances of missing any information.
Criteria of package inserts: The PIs were analyzed based on the following criteria: 1. Legibility. 2. Approved generic name of active ingredients. 3. Content of active ingredient per dosage form. 4. Generic names of other ingredients. 5. Therapeutic indications. 6. Posology and method of administration. 7. Contraindications. 8. Special warnings and precautions. 9. Drug interactions. 10. Pregnancy and lactation. 11. Pediatric and geriatric indications. 12. Special conditions and contraindications. 13. Effect on ability to drive and use machines. 14. Undesirable effects. 15. Drug dose. 16. Over dosage. 17. Pharmacokinetic information. 18. Storage information. 19. Instructions for use and handling. 20. Shelf life. 21. Date on which information was last updated. 22. Name and address of manufacturer/distributor. 23. Provision of full information on request should be highlighted. 24. Retail price of the drug. 25. References.
Scoring and grading of PIs: A total score of 25 was assigned to each, based on 25 criteria. Presence of information was scored as ‘1’ and absence was scored ‘0’. Total score was expressed in percentages. If a package insert met more than 20 criteria, it was graded as ‘A’; 10-20 criteria as ‘B’, and less than 10 as ‘C’.
Results
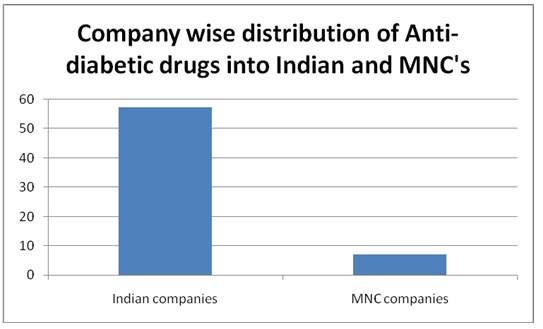
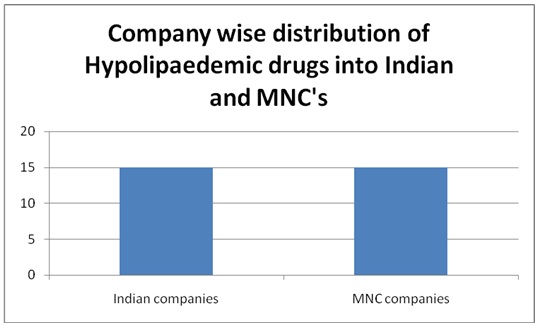
A total of 150 PIs were collected. Among them, 16 were repeated and were not considered for the study. A total of 134 PIs were analyzed. Out of them, 96 were from Indian companies and 38 from multinational companies. Among them, 64 were PIs for anti-diabetics, 40 were anti-hypertensives, and 30 were hypolipedemics. Out of 64 anti-diabetic PIs, 48 (75%) were oral and 16 (25%) were injectable preparations. All the anti-hypertensives and hypolipedemic PIs were of oral preparations. Out of 134 PIs, 31 (23.84%) belonged to Grade ‘A’ (including all injectable preparations) and remaining 76.86% to Grade ‘B’. The grading of PIs from Indian and multinational companies is shown in [Table/Fig-1]. It was observed that more PIs from Indian companies belonged to Grade A. None of the PIs belonged to Grade ‘C’. The company wise distribution of PIs of anti-diabetic, antihypertensives, and hypolipedemic drugs that were evaluated is shown in [Table/Fig-2,3and4] respectively. The percentage scores of the PIs are shown in [Table/Fig-5].
Grading of Package inserts in percentages
Company wise distribution of Anti-diabetic drugs
Company wise distribution of Anti-hypertensive drugs
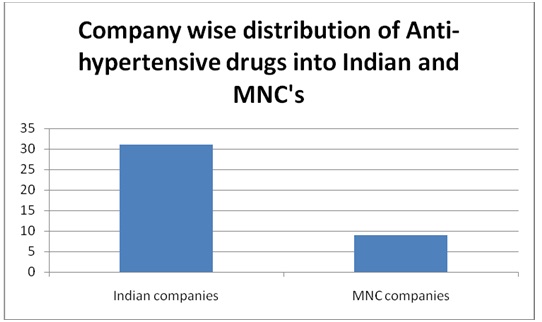
Company wise distribution of Anti-hypertensive drugs
Percentage score of PIs based on criteria lay down by Indian Drug and Cosmetic Rules, 1945
| Criteria number | Criteria | Total score of PIs in percentage (n= 134) |
|---|
| 1. | Legibility | 91.79 |
| 2. | Approved generic name of active ingredient | 100 |
| 3. | Content of active ingredient per dosage form | 100 |
| 4. | Generic names of other ingredients | 23.84 |
| 5. | Therapeutic indications | 100 |
| 6. | Posology and method of administration | 100 |
| 7. | Contraindications | 100 |
| 8. | Special warnings and precautions | 100 |
| 9. | Drug interactions | 100 |
| 10. | Pregnancy and lactation | 96.26 |
| 11. | Pediatric and geriatric indications | 88.80 |
| 12. | Special conditions and contraindications | 100 |
| 13. | Effect on ability to drive and use machines | 36.92 |
| 14. | Undesirable effects | 37.69 |
| 15. | Drug dose | 100 |
| 16. | Over dosage | 100 |
| 17. | Pharmacokinetic information | 100 |
| 18. | Storage information | 89.23 |
| 19. | Instructions for use and handling | 40.76 |
| 20. | Shelf life | 0 |
| 21. | Date on which information was last updated | 41.53 |
| 22. | Name and address of manufacturer/distributor | 100 |
| 23. | Provision of full information on request should be highlighted | 100 |
| 24. | Retail price of the drug | 0 |
| 25. | References | 0 |
Discussion
The information presented in the PIs is necessary for both the prescribers and the patients. A study done in private practitioners concluded that the majority of them (72%) found package inserts useful or extremely useful [10]. From patients point of view, PIs have an important impact on patient compliance and thus on the effectiveness of drug use [11]. Substantial regulatory efforts have been made in Europe, USA, Australia, and Saudi Arabia to improve the information content of PIs [12]. This is fortunate as a Saudi-based survey of over 2000 community pharmacy customers found that 88% of respondents claimed that they read the PIs or ask somebody to read it for them [13]. A study conducted at Palestine reported that 45% of consumers read the PIs [14]. In Indian scenario, due to inadequate doctor patient ratio, the accessibility to trained prescribers is difficult and physicians are not able to spend enough time with their patients. This gives rise to self-medication, medication errors, and adverse drug reactions. All these issues indicate the need for patient oriented PIs [15].
In this study, PIs of drugs for the most prevalent and chronic diseases like diabetes, hypertension and hyperlipedemia were analyzed to assess if they contained information according to Indian Regulatory Guidelines. It was observed that the PIs were inadequate in many aspects. The presentation of information, font size, and color was appropriate in 91.79%, generic names of other ingredients in 23.84%, information about the effect on ability to drive and use of machines in 36.92% PIs. Also, information about use in pregnancy and lactation was present in 96.26%, paediatric and geriatric indications in 88.80% and undesirable effects in 37.69% PIs. Again, storage information was adequate in 89.23%, instructions for use and handling in 40.76% and date on which information was last updated in 41.53% of PIs. However, information about shelf life, retail price of the drug and references were not present in any PIs. Information on shelf life is important as the drug that has passed its shelf life might still be safe for consumption but its quality cannot be guaranteed. This can lead to poor control of diseases like diabetes and hypertension. Also, patients should be well–informed about the retail price of the drugs and should be able to confer to references whenever required. It was, however, noted that there has been an overall improvement in the percentage of inserts containing information as compared to previous studies [16, 17].
From the above findings, it is suggested that the PIs must be optimized and tested by selected groups of experts prior to the approval of the drug. This will ensure the avoidance of the lack of information and will guide towards informed and better treatment outcomes. The supply of the PIs should be made mandatory in the package along with the drugs. The government should make strict rules to ensure that the pharmaceutical companies comply with the regulatory guidelines.
Conclusion
PIs have an important impact on the patients compliance and thus on the effectiveness of drug use. However, the need of the hour is to further refine the contents of the circulated PIs to make them complete, reliable, and up to date. This can be a step forward for ethical and effective dissemination of healthcare services in our growing society.
[1]. Ved JK, Package Inserts in India: Need for a RevisionInternational Journal of Pharma Sciences and Research 2010 1(11):454-56. [Google Scholar]
[2]. Food and Drug Administration, HHSRequirements on content and format of labeling for human prescription drug and biological productsFed Reg 2006 71:3921-97. [Google Scholar]
[3]. The Drugs and Cosmetics Act and Rules. Ministry of Health and Family Welfare, Government of India. 2003. p. 312. Available from: http://cdsco.nic.in/html/copy%20of%201.%20d & cact121.pdf. Accessed on March 15th, 2013 [Google Scholar]
[4]. Medication Adherence: Making the case for increased awareness. Available at: http://scriptyourfuture.org/wp-content/themes/cons/m/Script_Your_Future_Briefing_Paper.pdf. Accessed on 16th April, 2013 [Google Scholar]
[5]. Ramachandran A, Snehalatha C, Latha E, Vijay V, Viswanathan M, Rising prevalence of NIDDM in an urban population in IndiaDiabetologia 1997 40(2):232-37. [Google Scholar]
[6]. Ramachandran A, Das AK, Joshi AR, Yajnik CS, Shah S, Kumar KMP, Current status of diabetes in India and need for novel therapeutic agentsJAPI 2010 58:7-9. [Google Scholar]
[7]. Kokiwar PR, Gupta SS, Durge PM, Prevalence of Hypertension in a Rural Community of Central IndiaJAPI 2012 60:26-29. [Google Scholar]
[8]. Pandya H, Lakhani JD, Dadhania J, Trivedi A, The prevalence and pattern of dyslipidemia among Type 2 diabetic patients at rural based hospital in Gujarat, IndiaIndian Journal of Clinical Practice 2012 22(12):36-44. [Google Scholar]
[9]. Tayyem MM, Patient’s Safety Information Available On Drug Package Inserts Used In NeuroanesthesiaThe Internet Journal of Anesthesiology 2009 19(2)DOI: 10.5580/4c [Google Scholar]
[10]. Joubert PH, Skene D, Attitudes of private medical practitioners towards package inserts and other drug information sourcesS Afr Med J 1984 66:306-7. [Google Scholar]
[11]. Fuchs J, Hippius M, Schaefer M, Analysis of German package insertsInt J Clin Pharmacol Ther 2006 44(1):8-13. [Google Scholar]
[12]. Al-aqeel SA, Evaluation of medication package inserts in Saudi ArabiaDrug Healthcare Patient Saf 2012 4:33-38. [Google Scholar]
[13]. Bawazir SA, Abou-auda HS, Gubara OA, Public attitude toward drug technical package inserts in Saudi ArabiaJ Pharm Technol 2003 19:209-18. [Google Scholar]
[14]. Al-Ramahi R, Zaid AN, Kettana N, Sweileh W, Al-Jabi D, Attitudes of consumers and healthcare professionals towards the patient package inserts - a study in PalestinePharmacy Practice (Internet) 2012 Jan-Mar 10(1):57-63. [Google Scholar]
[15]. Morris LA, Patient Package Inserts: A new tool for patient educationPublic Health Reports 1977 92(5):421-24. [Google Scholar]
[16]. Lal A, Sethi A, Drug package inserts in IndiaAnn Pharmacother 1996 30:1041 [Google Scholar]
[17]. Shivkar YM, Clinical information in drug package inserts in IndiaJournal of Postgraduate Medicine 2009 55(2):104-07. [Google Scholar]