Pregnancy is a special physiological condition where drug treatment presents a special concern, because in this case, in addition to the health and life of mother, the health and life of her unborn child is also at stake. The drugs given to pregnant mothers for therapeutic purposes may cause serious structural and functional adverse effects in the developing child [1]. The concern on medication use during pregnancy and lactation has been influenced by historical events, including thalidomide crisis in the 1960s and the teratogenic effects discovered, related to the use of diethylstilboestrol in 1971. These events led the US Food and Drug Administration to establish strict regulations regarding drug labeling, the use of medications in pregnancy, requiring demonstrations on safety and efficacy of any drug before it became commercially available [2].
Although pregnancy is a physiological process, it still requires special care. Supplementary drug treatment, specially with iron, folic acid, calcium, vitamins and minerals, plays a pivotal role in the prevention of maternal and child mortality and morbidity [3].
The earlier studies done on drug use in pregnancy indicated that drug use in pregnancy was widespread [4]. Avoiding medications during pregnancy should be desirable, and may be dangerous, because some women enter pregnancy with medical conditions that require ongoing and episodic treatment (e.g. asthma, epilepsy, hypertension, etc). Also, during pregnancy, new medical problems can develop and old ones can get exacerbated (e.g. migraine, headache, anaemia), requiring pharmacological therapy. Failure in managing conditions like these may affect the health of both the mother and her infant [5].
Careful consideration of the benefit to the mother and the risk to the foetus is required, while prescribing drugs during pregnancy. Reducing medication errors and improving patient safety are of utmost importance [6]. Since it is very difficult to determine their effects on the foetus before marketing new drugs, due to obvious ethical reasons, most drugs are not recommended for use during pregnancy [7].
Therefore, medication safety information in pregnancy is actually obtained through case reports, epidemiological studies and animal studies [8]. Pharmacoepidemiological studies can measure the extent of prescription and teratogenic drug use in pregnant women. The studies conducted in developed countries where drug-prescribing practices are considered to be superior, have identified need for interventional measures aimed at rational prescription during the antenatal period [9].
Very few pharmacoepidemiological studies done on audits on the use of drugs during pregnancy have been conducted in India. So, it becomes important to examine the pattern of drug utilization in pregnancy, to check as to upto what extent there may be a room for improvement in the light of current knowledge [10]. Also, judicious use of drugs, adequate knowledge, positive approach and awareness towards/on drug use are mandatory prerequisites for good maternal and child health.
This study was planned to examine the pattern of drug utilization during pregnancy and to explore the knowledge, attitude and awareness towards/on drug use of antenatal mothers in tertiary care hospital setup.
Material and Methods
This study was started after getting approval from Institutional Ethics Committee (Ref no. BVDUMC/1554/2010-2011). It was an observational, cross-sectional study conducted from October 2010 to August 2012 in the Obstetrics-Gynaecology Department at Bharati Hospital, Pune, India. Study participants were pregnant women coming to the out-patients-Department and wards of Obstetrics-Gynaecology Department during the study period. All the participants gave written informed consents for inclusion in the study. Total of 501 such patients were enrolled in the study. Data collection was done for 3-4 days/week in the study period.
Study questionnaire: Questionnaires from previous studies were referred and modified for obtaining some additional information pertained to study population [11]. Validation of the questionnaire was done by conducting a pilot study on 60 participants and incorporating suggestions of experts after reviewing the study results. Information given by the patient was filled in the questionnaire by the investigator. It was cross verified from their case files and prescriptions to ensure that the information provided by patient was correct.
Study population was classified according to its educational status into illiterate, schooling and graduates. Study population was also classified according to its socioeconomic status by calculating per capita income/month [11].
Income class A =per capita Rs.500-999/month.
Income class B= per capita Rs. 1000-2500/month.
Income class C= per capita Rs.>2500/month.
Data Analysis: Statistical analysis was done using software, Stata, version 11. Results were expressed in percentage. Inter-group comparison (between groups according to trimester, IPD/OPD, educational status and economic status) was performed using Chi-square test. p value <0.05 was considered as statistically significant.
Results
[Table/Fig-1] shows that out of 501 pregnant women who attended the antenatal clinic and were admitted to Obstetrics- Gynaecology ward during their antenatal period, 35.5% were primigravida and 64.5% multigravida pregnant women. 289 were admitted to the ward during their antenatal period and 212 had come for their regular antenatal care (ANC) visits.
Characteristics of study population
| Parameter | Number of subjects (%) |
|---|
| Gravida | |
| Primigravida | 178 (35.52) |
| Multigravida | 323 (64.47) |
| IPD & OPD | |
| IPD | 289 (57.68) |
| OPD | 212 (42.31) |
| Duration of Pregnancy | |
| First TM | 79 (15.76) |
| Second TM | 141 (28.14) |
| Third TM | 281 (56.08) |
| Education status | |
| Illiterate | 15 (2.99) |
| Schooling | 372 (74.25) |
| Graduates | 114 (22.75) |
| Family income/capita/month | |
| Income class A | 35 (6.98) |
| Income class B | 163 (32.53) |
| Income class C | 303 (60.47) |
Most frequently prescribed drugs in first, second and third trimesters were iron, folic acid and calcium supplements which were category A drugs. Category B drugs which included analgesics (paracetamol, diclofenac sodium, ibuprofen, dicyclomine) antacids, antiulcer drugs (ranitidine, omeprazole, pantoprazole), antimicrobials (ampicillin, amoxicillin, cephalosporins, Metronidazole) were used for common ailments in pregnancy like nausea, upper respiratory tract infection (URTI), urinary tract infection (UTI), diarrhoea. Drugs of categories C and D, which included antihypertensives (nifedipine) anticonvulsants (phenobarbitone), insulin, isoxsuprine, antifungal drugs, digoxin, corticosteroids were more commonly used during 2nd and 3rd trimesters [Table/Fig-2]. Drugs used for chronic diseases–epilepsy, bronchial asthma, rheumatic heart disease, diabetes mellitus fell in categories C and D. The only category X drug (progesterone) was used for threatened abortions, missed abortions and preterm labour. It was mainly used in first and second trimesters in such IPD patients [Table/Fig-2 & 3].
Trimester-wise comparison of drug categories used during pregnancy
| Category of Drugs | First TM t.n.d.-241 n (%) | Second TM t.n.d.-477 n (%) | Third TM t.n.d.-1064 n (%) | p-value |
|---|
| A | 198 (82.15) | 336 (70.44) | 722 (67.85) | ***<0.0001 |
| B | 28 (11.61) | 94 (19.70) | 172 (16.16) | *0.02 |
| C | 4 (1.65) | 29 (6.07) | 129 (12.12) | ***<0.0001 |
| D | 1 (0.41) | 3 (0.62) | 16 (1.5) | 0.171 |
| X | 7 (2.90) | 7 (1.46) | 0 (0) | ***<0.0001 |
| Other Pathy Medications | 3 (1.24) | 8 (1.67) | 25 (2.34) | 0.450 |
Other pathy medications: Ayurvedic, herbal drugs
*p<0.05, ***p<0.001, t.n.d.-total number of drugs, TM-trimester.
P-value was calculated after comparing values between first, second and third trimester by using chi-square test with degree of freedom 2
IPD and OPD pattern of drug use in study population
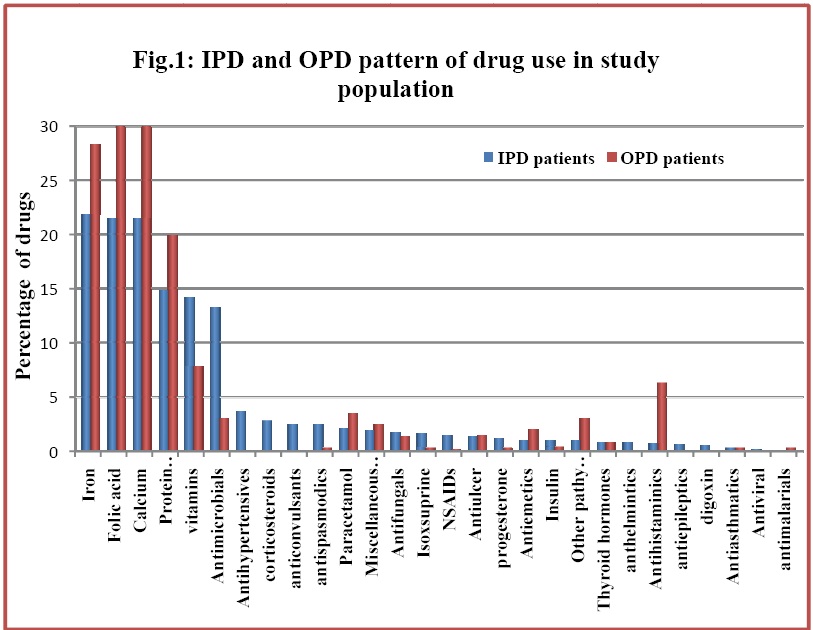
Category A drug use was significantly more in OPD patients in contrast to those in categories B, C and D, which were significantly more in IPD patients. Progesterone, the only Category X drug, was prescribed significantly more in IPD than in OPD patients. Use of other pathy drugs was seen significantly more in OPD patients than in IPD patients.
As seen in [Table/Fig-4] there were 40 pregnant patients who were suffering from various chronic illnesses and who were on drug treatment for these diseases even before pregnancy. Hypothyroidism was the most common of all the illnesses, followed by rheumatic heart disease, diabetes mellitus, epilepsy and bronchial asthma. We also encountered 1 patient each of rheumatic fever, beta-thalasaemia and chronic gastritis.
Distribution of patients with chronic illnesses in study population
| Chronic Illnesses in Study Population | Number of Patients | Treatment Given |
|---|
| Hypothyroidism | 15 | Thyroid hormone |
| Rheumatic heart disease | 11 | Inj.Benzathine penicillin & Digoxin |
| Diabetes Mellitus | 4 | Human Insulin |
| Epilepsy | 3 | Phenobarbitone, Carbamazepine, Lamotrigine |
| Bronchial asthma | 3 | Deriphylline |
| Rheumatic fever | 1 | Inj.Benzathine penicillin |
| Beta-thalasemia | 1 | Regular blood transfusions |
| Chronic gastritis | 1 | Pantoprazole |
| Diabetes + Hypothyroidism | 1 | Human insulin + Thyroid hormone |
Drug treatments and dosages were modified according to the illnesses and requirements which occurred during pregnancy. Most of the drugs were from categories A, B and C and only 2 drugs from category D were prescribed viz. phenobarbitone and carbamazepine for epilepsy.
Similarly [Table/Fig-5] shows that 258 study patients suffered from various acute illnesses during their antenatal period. These conditions were managed with mainly category A, B and C drugs. The category D drug used was phenobarbitone and X drug was progesterone.
Distribution of acute illnesses encountered during pregnancy in study population
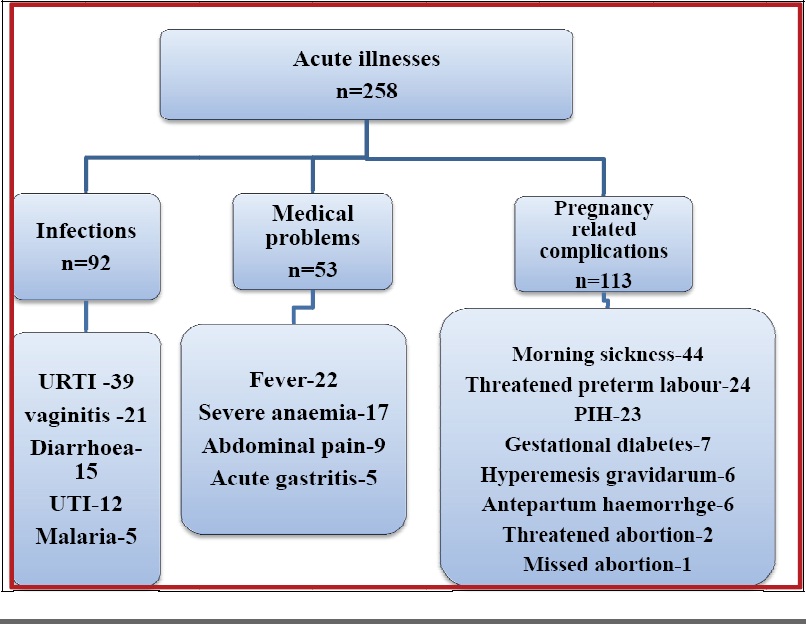
URTI-upper respiratory tract infection, UTI-urinary tract infection, PIH-pregnancy induced hypertension O.C. pills-oral contraceptive pills, IUCD-Intrauterine contraceptive devices.
[Table/Fig-6] shows the awareness of study population about various methods of contraception. Awareness on various common methods of contraception like oral contraceptive pills and barrier contraceptives was significantly more in literates than in illiterates (p<0.0001). Among literates, it was more in graduates than in schooling group. Graduates were more aware of IUCDs and injectable contraceptives than those in schooling group (p <0.0001), while illiterates were totally unaware about both these methods of contraception.
Awareness of study population about various methods of contraception
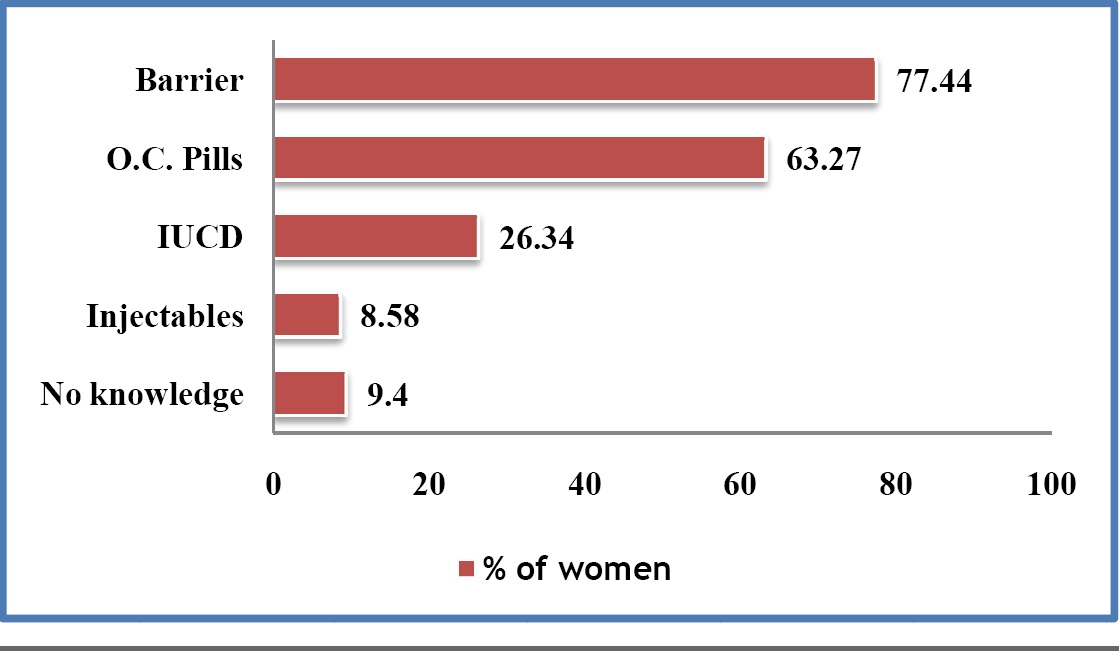
Also, awareness on these contraceptive methods was significantly more in higher socioeconomic group (income class C) than in lower socioeconomic groups (income classes A and B (p<0.0001). Study participants from income class A were totally unaware about injectable contraceptives.
Out of the study population, only 42% women knew about the safest method of contraception during pregnancy, which was barrier method. This knowledge was better among patients who had graduated (74.75%) than among those who were from schooling group (33.1%) and illiterates (20%).
There was no impact of education on number of antenatal visits attended and other pathy drug use in these pregnant women.
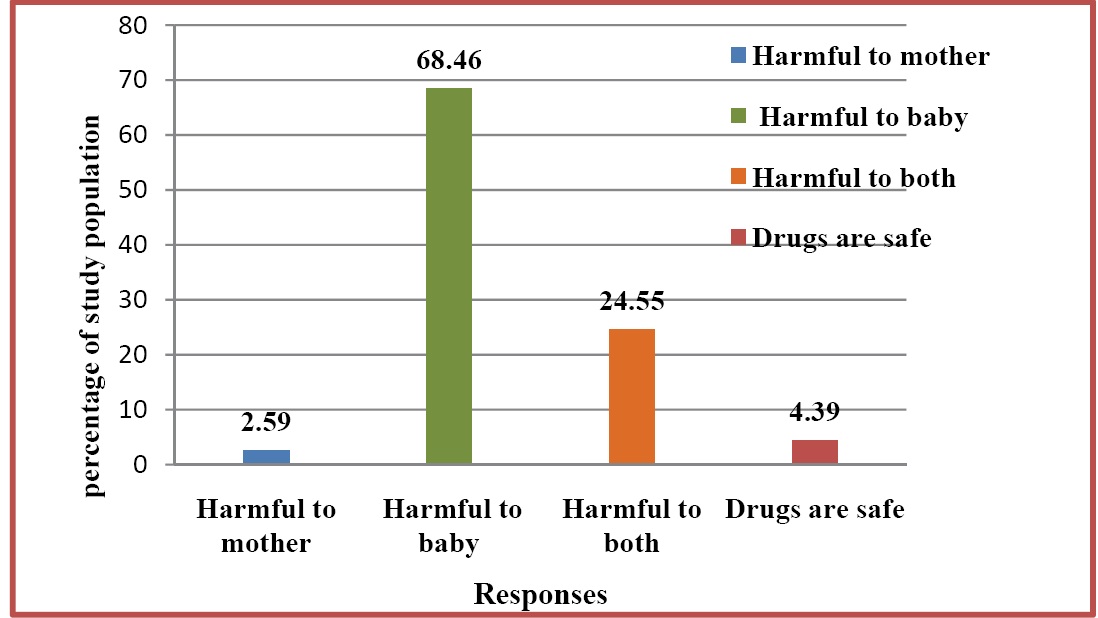
Majority of women believed that drug use in pregnancy could be harmful to baby, whereas only 2.59% of them believed that drug use during pregnancy could be harmful to mother. Some of them had a wrong belief that all drugs were safe in pregnancy [Table/Fig-7].
General understanding of study population about drug use in pregnancy
Discussion
The United States Food and Drug Administration has classified drugs into five categories (A, B, C, D and X), based on data from human and animal studies, to help physicians interpret the risk associated with drugs during pregnancy. The benefits of rational drug use during pregnancy relate to maternal health as well as to foetal development. Present study was an observational, cross sectional study, done to assess the status of current treatment practices in pregnant women and to study the patients’ perceptions of drugs consumed during pregnancy at a tertiary care hospital.
Iron, folic acid and calcium supplements were most commonly prescribed drugs in 90 to 95% women. They were started during first trimester in most patients, but in few of them, the initiation of these drugs was delayed due to late first antenatal visits. Among 501 patients, iron and calcium supplements were discontinued in 38.3% patients; temporarily in 150, whereas in 42 patients, it was permanent discontinuation. Reasons for temporary cases were mainly adverse effects of these drugs like diarrhoea, constipation, metallic taste and added up to the existing nausea and vomiting (morning sickness) of pregnancy.
Medication was continued as soon as adverse effects became less severe or disappeared. The reason for permanent discontinuation was mainly negligence and economic problems of patients. It was seen more in less educated (illiterate and schooling) group.
In our study, majority of drugs used during pregnancy belonged to USFDA category A, such as vitamins and mineral supplements. This was followed by category B drugs, which included paracetamol, diclofenac sodium, ibuprofen, antacids, dicyclomine, ranitidine, omeprazole, pantoprazole, ampicillin, amoxicillin, cephalosporins, metronidazole, methyldopa, which were prescribed for diseases encountered during pregnancy. There were some conditions where category C and D drugs like Nifedipine, insulin, clotrimazole, fluconazole, digoxin, chloroquine, isoxsuprine, betamethasone, phenobarbitone, and carbamazepine were prescribed to prevent complications caused by various disease conditions. The only Category X drug, Progesterone, was prescribed in cases of threatened miscarriages, missed abortions and preterm labour.
These results were encouraging as compared to findings of results obtained from other studies. A similar study conducted in north India reported that majority of the drugs used were from category-A, followed by those from category-B and category-D [11]. In Pakistan, a study conducted observed the drug-prescribing patterns during pregnancy in five tertiary care hospitals in different cities. They reported that apart from nutritional supplements, less than 1% women attending ANC clinic were prescribed teratogenic drugs [12]. Studies of Bratislava and Nitra reported that a vast majority of drugs prescribed during pregnancy belonged to category C [13]. The percentage of women who were prescribed teratogenic drugs was comparable to that reported in USA, where 5.8% of women received U.S. FDA category D or X drugs [14].
The benefits of medicine use during pregnancy are not restricted to the recovery of maternal health, but they also result in some advantages for the foetus as well, because maternal well-being is important for the optimal development of the foetus [15]. So, it is very important to treat various diseases in pregnancy. In present study, 40 patients were suffering from chronic illnesses which were managed by drugs, mainly by those from category B and C, except for antiepileptic drugs which belonged to category D. Similarly, for the acute problems encountered during pregnancy, category A, B and C drugs were more commonly prescribed. Among the other drugs used, Phenobarbitone was category D drug used for preeclampsia and progesterone was category X drug which was used for threatened abortions. This observation was similar to that of a prospective survey in south western Finland, where iron and vitamin supplementation were the most frequently used drugs, followed by analgesics, tocolytics, and drugs for chronic conditions and other symptoms and diseases encountered during pregnancy [16].
Also, a more recent study on prescription drug use, conducted in British Columbia, reported at least 1 prescription at some point in pregnancy for category D or X medicines [17]. In a Finnish study, 20.4% of women purchased at least one drug classified as potentially harmful during pregnancy and 3.4% purchased at least one drug classified as clearly harmful [18].
We also studied the use of other pathy drugs among pregnant women. Other pathy drugs which generally constitute herbal preparations are classified as dietary supplements by FDA and they are not regulated like conventional drugs. There is always a risk of interaction between herbal drugs and other medications, thus potentially making them less effective [11]. In our study, other pathy drugs were consumed by only 7% women, which constituted mainly Shatavari (renowned tonic for female reproductive system in Ayurveda), nutritional supplements and medicaments for cough. In a study from Norway, herbal drug use in 36% pregnant women was reported [19].
In India, easy availability of drugs, coupled with inadequate health services, leads to increased proportion of drugs used as self-medication [20]. Previous study conducted in north India reported that use of over the counter drugs as self medication was reported significantly more in graduates than in undergraduates, as well as more in higher socioeconomic classes than in lower ones [11]. Also, in a study from USA, over the counter medications (e.g.ibuprofen) that are contraindicated in pregnancy, were used at unexpectedly high rates during pregnancy [21]. But in our study, no patient reported taking any such over the counter medication other than some herbal medications.
Judicious use of drugs, adequate knowledge, positive approach and awareness towards/on drug use are mandatory prerequisites for good maternal and child health. This basic knowledge that inadvertent drug use in pregnancy can be dangerous to both mother and child and that drug intake can be dangerous at any time throughout pregnancy, was lacking in most of the our study participants.
Limitations of our study-1) There are chances that the actual exposure of the patients to teratogenic drugs could be different, because in a developing country like India, most of the pregnancies are unplanned. The pregnant women may be taking drugs before they know that they are pregnant. 2) Less number of women in first trimester may be due to the fact that in India; women mostly start seeking antenatal care in advanced pregnancy. 3) This was a cross sectional study, so we were unable to know the effects of drugs on pregnancy outcomes.
Conclusion
Present study revealed careful prescribing behaviour of physicians, with majority of safe drugs being prescribed during pregnancy. Harmful drugs were given only in situations like pregnancy associated complications, where benefits could outweigh risks. Patients’ educational and socio-economic statuses influenced their compliance for nutritional supplements prescribed during pregnancy and their awareness on common contraceptive methods. There is lack of awareness on safety of drugs in pregnancy in the study population. Hence, there is a need to educate and counsel women of child bearing ages regarding the advantages and disadvantages of drug use in pregnancy, as also regarding various methods of contraception.
Other pathy medications: Ayurvedic, herbal drugs
*p<0.05, ***p<0.001, t.n.d.-total number of drugs, TM-trimester.
P-value was calculated after comparing values between first, second and third trimester by using chi-square test with degree of freedom 2