Revised Ciprofloxacin Breakpoints for Salmonella: Is it Time to Write an Obituary?
Revathy Girish1, Anil Kumar2, Sadia Khan3, Kavitha R. Dinesh4, Shamsul Karim5
1 MSc, Department of Microbiology, Amrita Institute of Medical Sciences, Ponekara, Kochi-682041, Kerala India.
2 Clinical Associate Professor, Department of Microbiology, Amrita Institute of Medical Sciences, Ponekara, Kochi-682041, Kerala India.
3 Clinical Assistant Professor, Department of Microbiology, Amrita Institute of Medical Sciences, Ponekara, Kochi-682041, Kerala, India.
4 Clinical Professor, Department of Microbiology, Amrita Institute of Medical Sciences, Ponekara, Kochi-682041, Kerala India.
5 Professor & Head, Department of Microbiology, Amrita Institute of Medical Sciences, Ponekara, Kochi-682041, Kerala India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. V. Anil Kumar, Amrita Institute of Medical Sciences, Ponekkara, Kochi, Kerala-682041, India. Office:+91484 2801234 (Extn: 8015), Fax: +91484 2802020, Mobile: +919037401413
E-mail: vanilkumar@aims.amrita.edu
Objectives: To determine the minimum inhibitory concentration of ciprofloxacin among 50 blood stream isolates of Salmonella enterica.
Material and Methods: A total of 50 consecutive isolates of Salmonella enterica were tested for susceptibility to antimicrobials using the Kirby Bauer disk diffusion method. Minimum inhibitory concentrations were determined using Hi-Comb strips. All results were interpreted according to the CLSI guidelines.
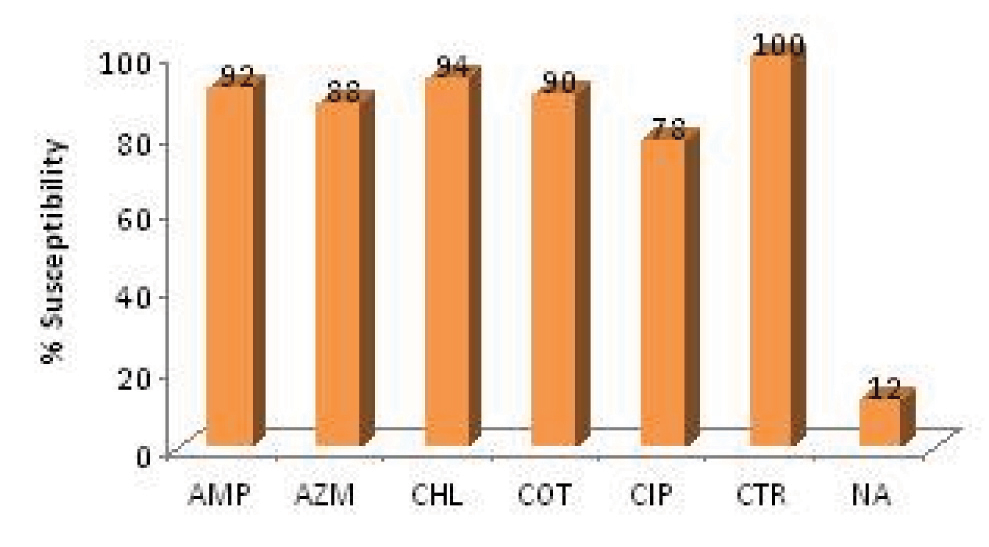
Results: Of the 50 isolates 70%were SalmonellaTyphi, 4% Salmonellaparatyphi A, 2% Salmonellaparatyphi B and the remaining 10% were identified only as Salmonella species. Using the CLSI 2011 breakpoints for disc diffusion, 86% (43/50) were resistant to nalidixic acid(NA), 22% (11/50) to ciprofloxacin, 12% to azithromycin, 6% to cotrimoxazole, 4% to ampicillin and 1% to chloramphenicol. The MIC50 and MIC90 of ciprofloxacin for S.Typhi were 0.181 μg/mL and 5.06 μg/mL respectively. While the same for S. paratyphi A was 0.212μg/mL and 0.228μg/mL respectively. None of the isolates were multi drug resistant and all were susceptible to ceftriaxone. Using the CLSI 2012 revised ciprofloxacin breakpoints for disc diffusion (>31mm) & MIC (<0.06 μg/mL), 90% (45/50) of these isolates were found to be resistant.
Conclusion: MIC’s of ciprofloxacin should be reported for all salmonella isolates and should be used to guide treatment. Blindly following western guidelines for a disease which is highly endemic in the subcontinent will spell the death knell of a cheap and effective drug in our armamentarium. Therefore it will be too premature to declare that “the concept of using ciprofloxacin in typhoid fever is dead!”
Salmonella typhi, Salmonella paratyphi A, Ciprofloxacin, Resistance, Breakpoints
Introduction
Enteric fever caused by Salmonella enterica serotype typhi and paratyphi A remains a major health problem in developing countries like India [1]. Drugs like cotrimoxazole, chloramphenicol, and ampicillin were previously used successfully to treat typhoid fever but gradually resistance to these agents emerged which lead to the use of fluoroquinolone as the antimicrobial agent of choice [2]. Subsequently, reduced susceptibility and in some cases frank resistance to ciprofloxacin began to be reported, increasingly [3–5]. Nalidixic acid (NA) resistance in salmonella has increased to 90% while susceptibility to ciprofloxacin is hovering around 85% [6]. Due to the ever increasing reports of treatment failure with fluoroquinolones in patients infected with Salmonella strains with reduced susceptibility to ciprofloxacin, there was an urgent need to revise ciprofloxacin breakpoints [7–9]. CLSI in 2012 has revised the breakpoints of ciprofloxacin from ≤1 μg/ml to ≤0.06 μg/ml for susceptible isolates making almost 90% of our isolates resistant to it [10].
The aim of our study was to determine the minimum inhibitory concentration of ciprofloxacin among 50 blood stream isolates of Salmonella enterica and interpret them in accordance to the revised CLSI breakpoints in 2012.
Material and Methods
Bacterial Culture and Identification
Blood stream isolates of Salmonella spp. were consecutively collected from patients admitted at Amrita Institute of Medical Sciences,Kochi between August 2010 to December 2011. The study was approved by the research and institutional ethics committee. Isolates were identified as Salmonella typhi and paratyphi A using automated VITEK 2 system (bioMérieux, Inc. St Louis, Mo), which is a 64-well plastic card containing 41 fluorescent biochemical tests that is more sensitive in detecting metabolic changes, including 18 enzymatic tests for aminopeptidases and –osidases,18 fermentation tests, 2 decarboxylase tests and 3 miscellaneous tests (urease, utilization of malonate, and tryptophane deaminase). The card is manually inserted in the VITEK 2 reader-incubator module (incubation temperature, 35.5°C), and every card is automatically subjected to a kinetic fluorescence measurement every 15 min. The results were interpreted by the ID-GNB database after the incubation period of 3 h. Confirmation was done using Salmonella polyvalent and type specific antisera. (Central research institute, Kasauli, India).
Antimicrobial Susceptibility Testing
Isolates were tested for susceptibility to antimicrobials using the Kirby Bauer disk diffusion method. Mueller Hinton agar plates were inoculated with a standardized inoculum of 0.5McFarland (approximately 108CFU/ml) over the entire surface.Antibiotic disks were dispensed to the agar surface with the forceps and incubated at 37oC for 16 to 18 hours in ambient air. Antibiotic disc contained ampicillin (10mcg), azithromycin (15mcg), chloramphenicol (mcg), cotrimoxazole (25mcg), ciprofloxacin (5mcg, ceftriaxone (30mcg)and nalidixic acid (30mcg) and diameter of inhibition zones were measured and interpreted according to CLSI guidelines (2011) [11]. (1) For azithromycin a diameter of ≤13mm was considered to be resistant [12].
Minimum inhibitory concentrations were determined using Hi-Comb strips (Hi-Media, India) which consists of a strip made of an inert material, with 8 extensions that carry the discs of 4mm, resembling the ‘tooth’ of a comb. Hi-Comb (based on principle of dilution & diffusion) consists of a gradient that covers a continuous range of 16 two-fold dilutions on two different strips part A&B. Part A strips consist of concentrations 0.01- 240μg/ml & Part B, 0.001-2μg/ml. Testing was done on Mueller Hinton agar plates inoculated with a standardized inoculum of 0.5 McFarland (approximately 108 CFU/ml) over the entire surface and Hi-comb strip was placed on medium in sterile condition. Plate was incubated for 24 hours at 37°C and zone of inhibition formed in an ellipse was observed. According to Hi-Comb MIC test, MIC value is the value at which the zone converges on the comb-like projections of the strips and not at the handle and zone of inhibition below the lowest concentration is to be considered (CLSI, 2011[11]).
Results
A total of 50 consecutive isolates of Salmonella enterica were collected from August 2010 to December 2011. The patients ranged in age from 20 years to 60 years (median, 40 years) Of these 72% were Salmonella typhi, 16% Salmonellaparatyphi A, 4% Salmonellaparatyphi B and the remaining 8%were identified only as Salmonella species.
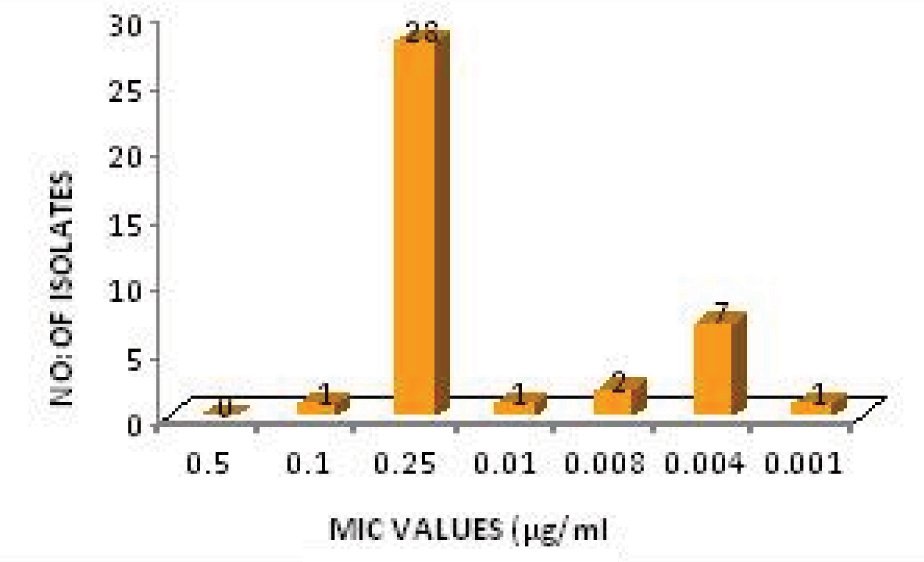
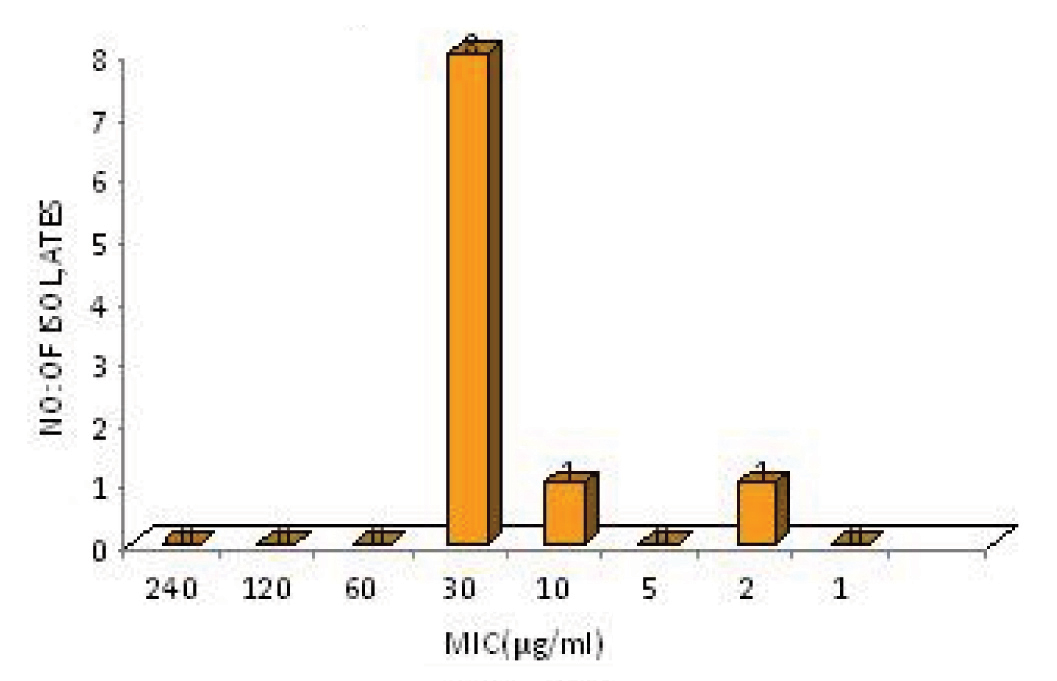
For Hi-Comb test the gradient remains stable after diffusion and the zone of inhibition created takes form of an ellipse. Antibiotic susceptibility was interpreted using the CLSI 2011 breakpoints for disc diffusion [Table/Fig-1]. The MIC50 and MIC90 of ciprofloxacin for S.typhi were 0.181μg/ml and 5.06μg/ml respectively, while the same for S. paratyphi A was 0.212μg/ml and 0.228μg/ml respectively [Table/Fig-2 and 3]. None of the isolates were multi drug resistant (MDR) and all were susceptible to ceftriaxone. Of the 11 isolates that were resistant to ciprofloxacin 80% had an MIC of 30 μg/ml while among the susceptible isolates 70% had an MIC of 0.25 μg/ml.
Antibiogram of Salmonella Isolates
(AMP, ampicillin; AZM, azithromycin; CHL, chloramphenicol; COT, cotrimoxazole; CIP, ciprofloxacin; CTR, ceftriaxone; NA, nalidixic acid)
MIC Distribution of ciprofloxacin susceptible salmonella isolates
MIC distribution of ciprofloxacin resistant Salmonella isolates
Using the CLSI 2012 revised ciprofloxacin breakpoints for disc diffusion (>31mm) & MIC (<0.06 μg/ml), 90% (45/50) of these isolates were found to be resistant ruling out fluoroquinolones as an option for treatment of typhoid fever. Similarly other guidelines have also revised the ciprofloxacin breakpoints for salmonella [Table/Fig-4].
Ciprofloxacin breakpoints for salmonella recommended in different guidelines.
| 2012 Guidelines (disc strength) | Disc diameter (mm) | MIC (μg/ml) | Comments |
|---|
| Resistant | Sensitive | Resistant | Sensitive |
|---|
| CLSI (5μg) | ≤20 | ≥31 | ≥1 | ≤0.06 | |
| EUCAST (5μg) | <19 | ≥22 | >1 | ≤0.5 | For ciprofloxacin, there is clinical evidence to indicate a poor response in systemic infections caused by Salmonella spp. with low level fluroquinolone resistance (>0.06mg/L). |
| BSAC (1μg) | ≤16 | ≥20 | >1 | ≤0.5 | Isolates with MICs greater than 0.06 mg/L should be reported as resistant. It is recommended that the ciprofloxacin MIC should be determined for all invasive salmonellae infections. |
For ciprofloxacin, there is clinical evidence to indicate a poor response in systemic infections caused by Salmonella spp. with reduced susceptibility to fluoroquinolones. Isolates with MICs greater than 0.06 mg/L should be reported as resistant. It is recommended that the ciprofloxacin MIC should be determined for all invasive salmonellae infections.
Antibiotic R> I S ≤ Disc content(μg) R ≤ I S ≥
Ciprofloxacin 1 1 0.5 1 16 17-19 20
Discussion
For empiric treatment of acute undifferentiated febrile illnesses in India, use of fluroquinolones is widespread due to its excellent activity against Salmonella and atypical pathogens [7,13]. However, Salmonella typhi isolates that are resistant to nalidixic acid and show decreased susceptibility to ciprofloxacin (0.125–1μg/ml) have become endemic in the Indian subcontinent [3–5]. This resistance to quinolones is caused by amino acid substitutions in the quinolone resistance–determining region of the DNA gyrase, subunit gyrA, gyrB or DNA topoisomerase IV(parC,parE) which are key targets of quinolones [14]. Single mutation in gyrA is said to be responsible for decreased susceptibility to ciprofloxacin whereas combination of 2 or more mutations in gyrA, gyrB, parC and parE makes them resistant [15]. Ciprofloxacin is concentrated in human monocytes and increases their bactericidal activity against intracellular bacteria which may explain why it is still effective in achieving clinical cure in patients with salmonella infections, which is intracellular. Ciprofloxacin brings about concentration dependent killing and is 30% protein bound. Keeping the pharmacokinetic and pharmacodynamic properties for Gram negative bacteria in mind (AUC/MIC>125), a higher dose of 750mg twice daily has been successfully used in treating enteric fever [6,16]. This holds true only if the isolate has an MIC <0.176 μg/ml as the AUC at this dose would be 22. Prior to 2012 the NA and ciprofloxacin susceptibility among our Salmonella typhi isolates were 0% and 84% in 2009; 1.2% and 67% in 2010 and 10% and 85% in 2011 respectively. CLSI has revised breakpoints [Table/Fig-4] for ciprofloxacin from <1 μg/ml in 2011 to <0.06 μg/ml in 2012 for susceptible Salmonella isolates making almost 90% of our isolates resistant to it. Prevalence of NA resistance is rising in India from 51% in 2006 [17] to 87.8% in 2012 [18]. A steady fall in the prevalence of multi drug resistant (MDR) Salmonella isolates from 94% in 1989-91 [19] to 39% in 2006 [20], has also been documented. Rai et al., found only 5% of Salmonella typhi and none of the paratyphi A isolates to be MDR in a study from Lucknow [18]. Mandal et al., found only 2.6% resistance to ciprofloxacin among their Salmonella isolates in Kolkata [21]. Gupta et al., reported 92.5% resistance to NA and 100% susceptibility to ciprofloxacin but none of the salmonella isolates had an MIC of <0.062 therefore making them all resistant if the revised CLSI guidelines were to be used for interpretation [22]. Nagshetty et al., from Karnataka reported a 10% prevalence of MDR and resistance to ciprofloxacin was only 4.2% [23]. Other commonly used guidelines like European committee on antimicrobial susceptibility testing (EUCAST) and British society of antimicrobial chemotherapy (BSAC) have also revised their breakpoints in line with CLSI [Table/Fig-4]. With ciprofloxacin MIC50 of 0.181μg/ml and 0.212μg/ml for S.typhi and paratyphi A respectively, using it for treatment should be based on actual MIC of the isolates. Considering the fact that ciprofloxacin is still being successfully used to treat enteric fever in spite of the creeping MIC, studies need to be done to address the extent of clinical failure seen in Indian patients. The absence of MDR isolates and increased susceptibility to chloramphenicol, cotrimoxazole ampicillin and azithromycin argues well with their use as an alternative to ciprofloxacin for treating enteric fever.
Conclusion
MIC’s of ciprofloxacin should be reported for all Salmonella isolates and should be used to guide treatment. Blindly following western guidelines for a disease which is highly endemic in the subcontinent will spell the death knell of a cheap and effective drug in our armamentarium. Therefore it will be too premature to declare that “the concept of using ciprofloxacin in typhoid fever is dead!”
For ciprofloxacin, there is clinical evidence to indicate a poor response in systemic infections caused by Salmonella spp. with reduced susceptibility to fluoroquinolones. Isolates with MICs greater than 0.06 mg/L should be reported as resistant. It is recommended that the ciprofloxacin MIC should be determined for all invasive salmonellae infections.Antibiotic R> I S ≤ Disc content(μg) R ≤ I S ≥Ciprofloxacin 1 1 0.5 1 16 17-19 20
[1]. Crump JA, Mintz ED, Global trends in typhoid and paratyphoid feverClin Infect Dis 2010 50(2):241-6. [Google Scholar]
[2]. Wain J, Kidgell C, The emergence of multidrug resistance to antimicrobial agents for the treatment of typhoid feverTrans R Soc Trop Med Hyg 2004 98(7):423-30. [Google Scholar]
[3]. Parry CM, Thuy CT, Dongol S, Karkey A, Vinh H, Chinh NT, Suitable disk antimicrobial susceptibility breakpoints defining Salmonella enterica serovar Typhi isolates with reduced susceptibility to fluoroquinolonesAntimicrob. Agents Chemother 2010 54:5201-08. [Google Scholar]
[4]. Matheson N, Kingsley RA, Sturgess K, Aliyu SH, Wain J, Dougan G, Ten years experience of Salmonella infections in Cambridge, UKJ. Infect 2010 60:21-25. [Google Scholar]
[5]. Le TA, Fabre L, Roumagnac P, Grimont PA, Scavizzi MR, Weill FX, Clonal expansion and microevolution of quinolone-resistant Salmonella enterica serotype Typhi in Vietnam from 1996 to 2004J Clin Microbiol 2007 45(11):3485-92. [Google Scholar]
[6]. Rodrigues C, Kumar NJ, Lalwani J, Mehta A, Ciprofloxacin breakpoints in enteric fever—time to revise our susceptibility criteriaIndian J Med Microbiol 2008 27(3):276 [Google Scholar]
[7]. Jesudason MV, Malathy B, John TJ, Trend of increasing levels of minimum inhibitory concentration of ciproß oxacin to Salmonella typhiIndian J Med Res 1996 103:247-9. [Google Scholar]
[8]. Rupali P, Abraham OC, Jesudason MV, John TJ, Zachariah A, Sivaram S, Mathai D, Treatment failure in typhoid fever with ciprofloxacin susceptible Salmonella enterica serotype TyphiDiagn. Microbiol. Infect. Dis 2004 49:1-3. [Google Scholar]
[9]. Lindgren MM, Kotilainen P, Huovinen P, Hurme S, Lukinmaa S, Webber MA, Reduced fluoroquinolone susceptibility in Salmonella enterica isolates from travelers, FinlandEmerg. Infect. Dis 2009 15:809-812. [Google Scholar]
[10]. Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement;CLSI document M100-S22. Clinical and Laboratory Standards Institute,Wayne, PA [Google Scholar]
[11]. Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement; CLSI document M100-S21. Clinical and Laboratory Standards Institute,Wayne, PA [Google Scholar]
[12]. Butler T, Sridhar CB, Daga MK, Pathak K, Pandit RB, Khakhria R, Treatment of typhoid fever with azithromycin versus chloramphenicol in a randomized multicentre trial in IndiaJ Antimicrob Chemother 1999 44(2):243-50. [Google Scholar]
[13]. Kim DM, Neupane GP, Jang SJ, Kim SH, Lee BK, Invitro efficacy of the combination of ciprofloxacin and cefotaxime against nalidixic acid resistant Salmonella enterica serotype TyphiInt. J. Antimicrob. Agents 2010 36(2):155-58. [Google Scholar]
[14]. Le Gall S, Desbordes L, Gracieux P, Saffroy S, Bousarghin L, Bonnaure-Mallet M, Distribution of mutation frequencies among Salmonella enterica isolates from animal and human sources and genetic characterization of a Salmonella Heidelberg hypermutatorVet. Microbiol 2009 137(3-4):306-12. [Google Scholar]
[15]. Neupane GP, Kim DM, Kim SH, Lee BK, In vitro synergism of ciprofloxacin and cefotaxime against nalidixic acid-resistant Salmonella enterica serotypes Paratyphi A and Paratyphi BAntimicrob Agents Chemother 2010 54(9):3696-701. [Google Scholar]
[16]. Gupta LK, Randhawa VS, Ciprofloxacin breakpoints in enteric fever: time to revise our susceptibility criteriaIndian J Med Microbiol 2008 26(4):406 [Google Scholar]
[17]. Capoor MR, Nair D, Hasan AS, Aggarwal P, Gupta B, Typhoid fever: Narrowing therapeutic options in IndiaSoutheast Asian J Trop Med Public Health 2006 37(6):1170-4. [Google Scholar]
[18]. Rai S, Jain S, Prasad KN, Ghoshal U, Dhole TN, Rationale of azithromycin prescribing practices for enteric fever in IndiaIndian J Med Microbiol 2012 30(1):30-3. [Google Scholar]
[19]. Chitnis V, Chitnis D, Verma S, Hemvani N, Multidrug-resistant Salmonella typhi in IndiaLancet 1999 354(9177):514-15. [Google Scholar]
[20]. Manchanda V, Bhalla P, Sethi M, Sharma VK, Treatment of enteric fever in children on the basis of current trends of antimicrobial susceptibility of Salmonella enterica serovar typhi and paratyphi AIndian J Med Microbiol 2006 24(2):101-6. [Google Scholar]
[21]. Mandal S, Debmandal M, Pal NK, Antibiotic resistance of Salmonella enterica serovar Typhi in Kolkata, India, and in vitro experiments on effect of combined chemotherapyScientificWorldJournal 2012 (2012):454-59. [Google Scholar]
[22]. Gupta V, Kaur J, Chander J, An increase in enteric fever cases due to Salmonella Paratyphi A in & around ChandigarhIndian J Med Res 2009 129(1):95-8. [Google Scholar]
[23]. Nagshetty K, Channappa ST, Gaddad SM, Antimicrobial susceptibility of Salmonella Typhi in IndiaJ Infect Dev Ctries 2010 8 4(2):70-3. [Google Scholar]