Tuberculosis (TB) is one of the major infectious bacterial diseases. It affects one third of the world’s population. In 1990 World Health Organization (WHO) report on Global burden of the disease ranked TB as seventh most morbidity causing disease in the world and expected to continue at same position till 2020. While pulmonary tuberculosis is the most common presentation, extra-pulmonary tuberculosis (EPTB) is also on increase world over. EPTB constitutes about 15 to 20 per cent of all cases of tuberculosis in immunocompetent patients and accounts for more than 50 per cent of the cases in HIV-positive individuals [1]. Among EPTB, Pleural tuberculosis (PTB) is more common. Except for tubercular emphysema, the bacterial load in the pleural effusion of tubercular pleurisy is very low. Owing to this fact, the reliability of conventional methods of laboratory diagnosis is very low. AFB smear lacks sensitivity and culture is time consuming and moreover negative in 80-90% of cases [2].
Methods like Adenosine Deaminase (ADA) activity, although rapid and being low in cost, gives cross reactivity with other infectious disorder therefore is not considered as specific marker for PTB [3].
As an alternative to these classical methods, new nucleic acid-based technologies like Polymerase chain reaction (PCR) showed promises as more rapid, sensitive and specific means of detection and identification of mycobacteria. However variable sensitivities and specificities have been obtained with different PCR methods depending upon area of genome that is amplified and the technique used for DNA extraction [4–6]. In the present study we evaluated nested PCR (nPCR) using IS6110 primers specific for Mycobacterium tuberculosis-complex (MTB-complex) and compared the results with conventional methods like smear and culture and biochemical tests like ADA activity.
Material and Methods
50 non-repeated pleural samples from the patients having a strong clinical or radiological evidence of tuberculosis and not receiving anti tubercular treatment were included in the study. The study was carried out in the Department of Microbiology and Biochemistry from September 2009 to May 2012.
All the pleural fluid samples were apportioned for conventional bacteriological techniques and for PCR procedure. The samples were centrifuged at 3000 rpm for 30 minutes. The supernatant obtained after concentration was used for ADA activity assay by the colorimetric method of Guisti based on indirectly measuring the formation of ammonia produced when adenosine deaminase acts with excess of adenosine. The cut-off level taken for considering a patient to be positive for M. tuberculosis using ADA levels was 47.3 IU/118 [7]. The deposits were processed by Ziehl-Neelsen (ZN) staining for acid fast bacilli (AFB), culture on Lowenstein-Jensen (LJ) medium and PCR.
Polymerase Chain Reaction
Since pleural fluids are paucibacillary in nature the detection of M. tuberculosis was done using nested PCR technique.
Extraction of DNA from clinical samples
A portion of material obtained after concentration was then further processed for DNA extraction using commercially available kit, Amplification Reagent Set for Mycobacterium tuberculosis, Bangalore Genei, Bangalore, India. The guidelines of the manufacturer of the kit were followed.
Nested polymerase chain reaction (nPCR)
Single tube nPCR using primers targeting IS6110 gene sequence of MTB was performed using PCR thermal cycler. Nested primers (synthesized by Bangalore Genei, Bangalore, India) included primers for 220 bp IS region of MTB-complex DNA sequence which was amplified in first round of amplification. In second step nested primers were added to further amplify a 123 bp amplification product.
Gel electrophoresis of amplified DNA
Amplified DNA was subjected to electrophoresis in 2.5% agarose gel containing ethidium bromide. The gel was run at 110 volts and then visualized under UV transilluminator. The results were documented.
Throughout the PCR processing, recommended stringent precautions were followed and the results were evaluated in the light of the performance of appropriate positive and negative controls, to avoid cross-contamination and false positive reactions.
Other Routine laboratory investigations including Hemogram, ESR, X-ray chest and Mantoux test were also done for all patients. All the necessary clinical details were also taken.Known treated positive TB cases, failure cases, defaulter cases, relapse cases were not considered for this study.
Statistical Analysis
The sensitivity of the conventional tests and PCR assay was calculated keeping culture as gold standard and the significance of difference was determined by proportion test; probability value of 0.05 was taken as significant value (p < 0.05). The study was approved by ethical committee of the institute.
Results
Of the total 50 clinical samples obtained from patients with suspected TB, only 3 samples were positive by smear examinations, 5 were positive with the gold standard, i.e. culture and 29 samples were positive with PCR. 16 out of 29 PCR positive samples and 5 out of the 21 PCR negative samples also showed high ADA activity [Table/Fig-1].
Results of conventional bacteriological tests, ADA and PCR
| Diagnostic variables | ZN Smear positive | Culture positive | ADA positive | PCR positive |
|---|
| Positive | 3 | 5 | 21 | 29 |
| Sensitivity | 60.00% | - | 80.00% | 100.00% |
| Specificity | 100.00% | - | 62.22% | 56.67% |
| Pearson x2 | 0.543 | - | 13.306 | 25.668 |
| p value | 0.461NS | - | <0.001** | <0.001** |
(NS: p > 0.05; Not Significant; ** p < 0.001; highly significant. Total samples (pleural fluid) = 50, Culture to be taken as gold standard)
Among the 29 patients with a positive PCR results for MTB, in 26 patients, apart from clinical history suggestive of TB also had one or more of the following parameters like pleural fluid examination positive for AFB (smear or culture), radiological evidence of TB and response to anti-tuberculosis therapy. None of the patients who were pleural fluid PCR negative had positive sputum smear for AFB, or a positive mycobacterial culture or radiological evidence of TB.
Based on the above results, the sensitivity and specificity of the PCR assay were calculated for the diagnosis of active TB; the overall sensitivity of the PCR assay was 100 per cent, specificity 56.65% and p = 0.001which is highly significant which was calculated taking culture on LJ medium as gold standard [Table/Fig-1and 2].
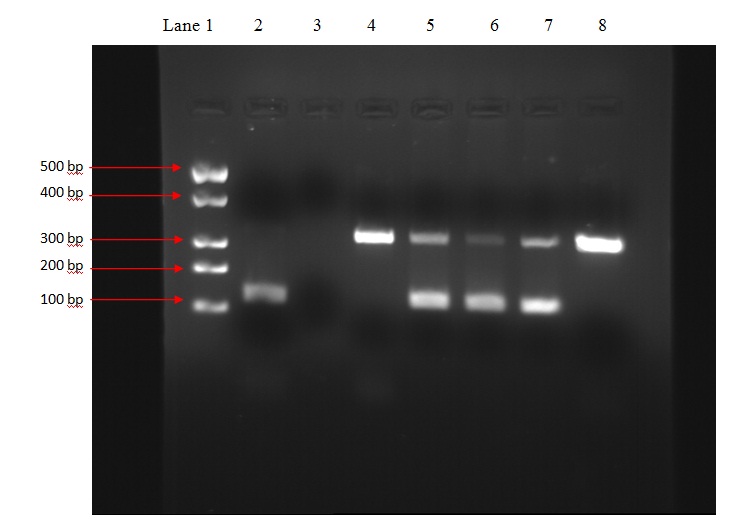
Showing positive results under UV Transilluminator
Above table show the comparison of different laboratory parameter with nPCR in suspected cases of TB.
Lane 1 DNA molecular weight marker (500-100bp above downwards).
Lane 2 Positive control with band at 123bp.
Lane 3 Negative control with no band.
Lane 4 Sample 1 showing band at 340bp (internal control) only, result is negative.
Lane 5 Sample 2 showing band at 340bp and 123bp, result is positive.
Lane 6 Sample 3 showing band at 340bp and 123bp, result is positive.
Lane 7 Sample 4 showing band at 340bp and 123bp, result is positive.
Lane 8 Sample 5 showing band at 340bp only, result is negative.
Discussion
Early and accurate diagnosis of tuberculosis is important for its effective management. Due to paucibacillary nature of pleural fluid, the conventional methods such as culture and smear often fail to give results.
Smear examination for AFB is a rapid method but insensitive and it requires sufficient number of acid fast bacteria to be present (104 organisms/ml) in the specimen. In our study also only 3 specimens were positive for AFB by smear examinations.
Diagnosis by culture, the gold standard, is specific but less sensitive for extra-pulmonary tuberculosis and also takes a long time. Only 5 specimens showed growth on LJ medium, in our study.
This study was done to evaluate the efficacy of nested PCR assay using primers targeting IS6110 to detect MTB DNA in pleural fluid in correlation with conventional bacteriological techniques and biochemical investigations. Diagnosis by PCR took two days in our study and 29 samples showed positive results. The use of PCR on pleural fluid for the diagnosis of TB has been reported previously but the sensitivity of PCR is variable ranging from 11 to 81 per cent [8–12].
We have used the gold standard, culture on LJ medium, to calculate the sensitivity and specificity of PCR and the overall sensitivity of the PCR assay was 100 per cent, specificity 56.65%. Among the 50 samples, the 5 samples which were positive by culture were also positive with PCR, had clinical evidence of disease and also showed positive response to ATT. But among these 5 specimens only 3 showed high ADA activity. Thus PCR has been better in sensitivity and specificity as compared to ADA levels. Similarly among the 21 PCR negative samples, 4 of the samples showed high ADA activity.
In our study negative PCR results were seen in 21 patients with PTB which could be either due to presence of inhibitors that are particularly high in pleural fluids or due absence or the presence of fewer copies of target sequence IS6110 in some strains of M. tuberculosis related to geographical origin has been already reported in the studies by Das et al., and DS Chauhan et al., [13, 14].
Studies conducted by Parandaman V et al., [15], Tan J et al., [16], Takagi N et al., [17] and Jatana SK et al., [18] have also shown similar results where sensitivity near 100% and specificity varied from 75-90%. These studies also have been carried out on targeting the insertion sequence IS6110.
Conclusion
To conclude nPCR assay targeting IS6110 gene sequence can be a useful tool in the establishment of diagnosis of PTB where there is strong clinical suspicion and conventional techniques are negative. However a combined analysis of nPCR, ADA activity and other laboratory parameters can be helpful to achieve more rapid and accurate diagnosis.
(NS: p > 0.05; Not Significant; ** p < 0.001; highly significant. Total samples (pleural fluid) = 50, Culture to be taken as gold standard)