At least one episode of vaginal candidiasis (VC) is experienced by 75% of women during their childbearing years[1]. Majority of VC cases are caused by C. albicans [2]. However, episodes caused by non-albicans Candida spp (NAC) are increasing [3]. The widespread reports on fluconazole resistance in Candida species and increased prevalence of non-albicans Candida spp. necessitate studies of Candida species distribution and antifungal susceptibility pattern in VC [4]. The clinical use of 5-flucytosine is limited, in part, by the perceived high frequency of primary resistance and the rate at which secondary resistance develops during treatment. However, large scale studies have shown primary resistance to C.albicans to be in the range of 3-6.5% [5]. Primary resistance to 5-flucytosine (5FC) occurs by decreased activity of either cytosine deaminase or UPRTase due to mutations in the genes, FCY1 or FUR1 [6,7].The study of Dodgson AR et al., showed single mutation at position 301 in FUR1 gene, responsible for 5FC resistance in C.albicans. In the present study, an attempt was made to detect the presence of mutant FUR1 gene in 5FC resistant Candida spp.
This cross sectional study was conducted in the Department of Microbiology in a tertiary care hospital in Chennai, south India, from July to December 2008. Two hundred sexually active women (18-45 years) with complaints of white discharge per vaginum, itching and vaginal discomfort were included in the study. Patients on antibiotics, those taking OCP, pregnant patients and those who were menstruating were excluded from the study. A detailed history regarding genital hygiene, use of tight fitting synthetic/ nylon underclothes and other relevant medical history were taken from all participants. Informed consents were obtained from the participants. Ethical committee approval was obtained. A thorough gynaecological examination was done and the clinical findings were recorded. Two vaginal swabs were collected- one for direct smear and the other swab for culture. Culturing was done on Sabouraud’s dextrose agar and CHROM agar Candida medium (HiMedia labs, India). 72 Candida isolates were obtained and they were speciated by standard morphological and biochemical tests. Antifungal susceptibility was performed by disk diffusion method according to CLSI M44-A document [9] using itraconazole (10μg), 5FC (10μg) and fluconazole (10μg) antifungal disks. C. krusei ATCC 6258 was used as control strain. 5FC resistant isolates were subjected to polymerase chain reaction (PCR) for detection of FUR1 genes.
Detection of FURI Genes
(i) DNA extraction [10] All 5FC resistant Candida strains were grown on Sabouraud’s dextrose agar for 24 hours. Cells were harvested and genomic DNA was extracted. A thick suspension of the growth (200 μl) was mixed with 300 μl of lysis buffer (10 mMTris-Hcl, 10mM EDTA, 50mM Nacl, 0.2% sodium dodecyl sulfate). 25 μl Proteinase K was then added to make it to a final concentration of 20 mg/ml, vortexed, incubated for 30 min at 56oC, boiled for 4 min and subsequently kept in ice for 5 min, centrifuged at 4oC for 5 min at 10,000 rpm. To the supernatant, equal volume of phenol: chloroform (1:1) mixture was added, mixed and centrifuged for 5 min at 4oC at 10,000 rpm. The aqueous phase was transferred to a new Eppendorf tube and double the volume of absolute alcohol was added, centrifuged for 5 min at 4oC at 10,000 rpm. Ethanol was discarded and to the pellet, 100 μl of 70% ethanol was again added and it was discarded immediately. The pellet was dried and suspended in 20μl of Tris-EDTA buffer (pH-8).
(ii) Polymerase chain Reaction(PCR): 10X PCR buffer 2.5 μl, dNTP 100 mM 2.5 μl, DNA 1 μl, Forward primer 2.5 μl, reverse primer 2.5 μl, Taq polymerase 5 U/ml 2 μl, milli Q water 12 μl were taken in a PCR tube. PCR was carried out in a Bioneer thermal cycler in the following steps:
Initial denaturation for about 5 min at 94oC
Each cycle had 2nd denaturation at 94oC for 1 minute.
Annealing at 57oC for 1 min
Extension at 72oC for 1 min
Thirty five cycles were performed and a final extension was done for 7 min at 72oC. Samples were cooled to 4oC. Primer sequences6 used were:
FUR 1 F1 – (5’ CGCAA CC TGA TTTTGT CCATA)
FURI RI – (5’ ATCGGAAGAATATCAT GAAAATCC)
(iii) Electrophoresis and Gel Documentation: After the reaction, 10μl of amplified products were electrophoresed on 2% agarose gel containing 0.5 μg/ml of ethidium bromide for 30 min at 80V in TBE buffer (0.089 M Tris –HCl, 0.089 M boric acid, 0.002 M EDTA (PH 8.4). The product size (340bp) was verified by comparison with hinf 1 digested pBR – 322 molecular size marker. Agarose gel was viewed in a UV transilluminator and also in UV gel documentation unit and photographed.
Results
Prevalence of vaginal candidiasis was 36%. A total of 72 Candida isolates were obtained from 200 symptomatic sexually active women. The results have been shown in [Table/Fig–1,2,3,4,56and7].
Age wise distribution of Vaginal Candidiasis
| Age Group (years) | No. of Cases Studied | No. of Cases Positive | Percentage |
|---|
| < 20 | 10 | 1 | 1.38% |
| 21 – 30 | 74 | 20 | 27.7 % |
| 31 – 40 | 86 | 43 | 59.7 % |
| > 40 | 30 | 8 | 11.1% |
Vaginal Candidiasis and Genital Hygiene
| Genital Hygiene | No. of Cases Studied | No. of Positive Cases | Percentage | p–value |
|---|
| Satisfactory | 153 | 50 | 32.67% | p<0.01 significant |
| Poor | 47 | 22 | 46.8% |
Vaginal Candidiasis and use of Tight fitting synthetic / Nylon Underclothings
| Tight fitting synthetic/Nylon Underclothings | No. of Cases Studied | No. of Positive Cases | Percentage | p–value |
|---|
| User | 58 | 30 | 51.75 % | p<0.01 significant |
| Non- User | 142 | 42 | 29.57 % |
Comparison of different methods for speciation of Candida isolates
| Species | Carbohydrate Assimilation test | CHROMagar Candida |
|---|
| C. albicans | 35 | 35 |
| C. glabrata | 21 | 21 |
| C. tropicalis | 8 | 8 |
| C. krusei | 4 | 4 |
| C. kefyr | 2 | - |
| C. parapsilosis | 2 | - |
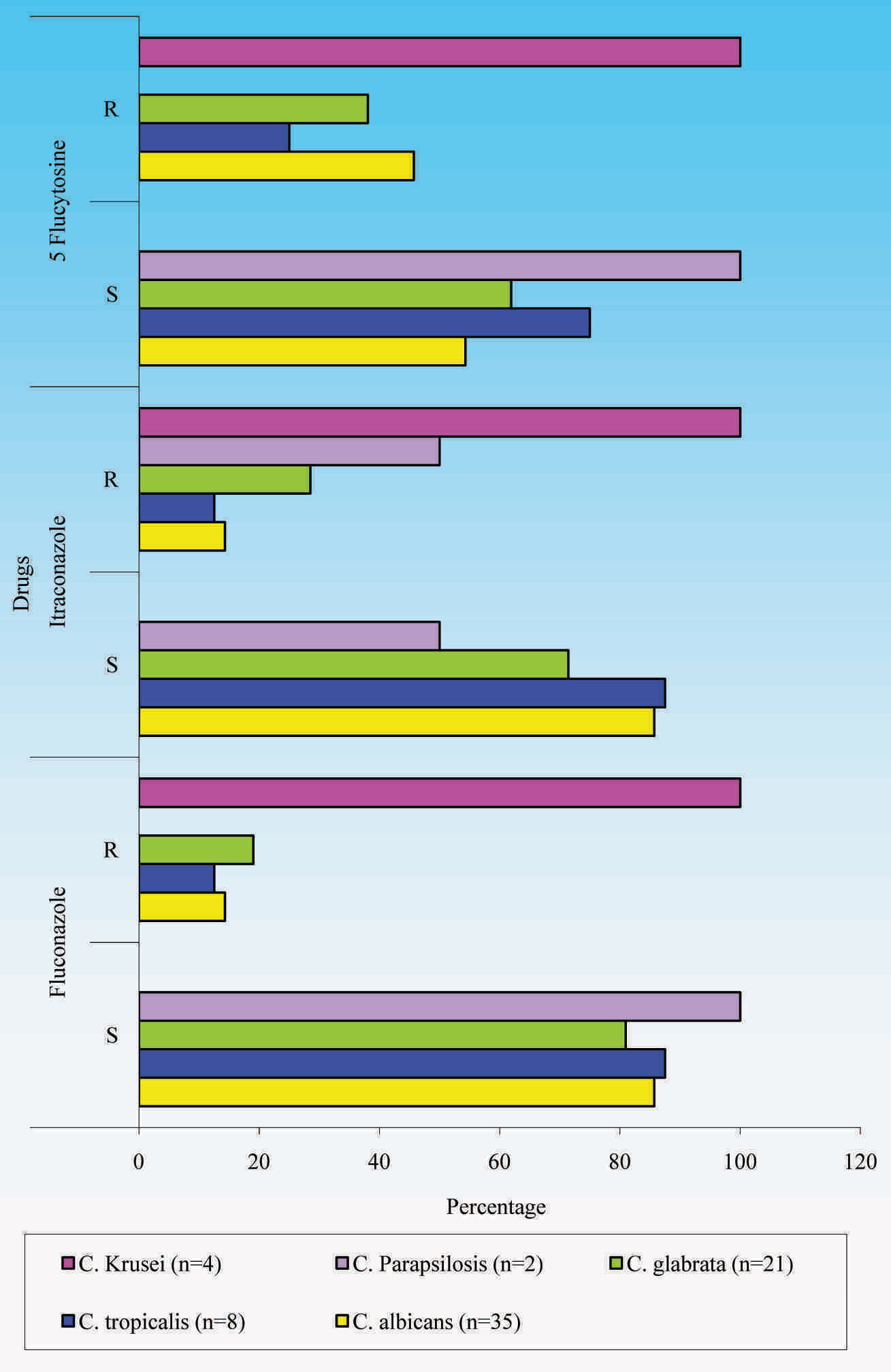
Antifungal Susceptibility Pattern of 72 Candida isolates
| No. of Isolates | Fluconazole (%) | Itraconazole (%) | 5-Flucytosine (%) | p–value |
|---|
| Sensitive | 58 (80.55%) | 55 (76.38%) | 42 (58.33%) | p<0.01 significant |
| Resistant | 14 (19.44%) | 17 (23.61%) | 30 (41.66%) |
Antifungal Susceptibility Pattern of Candida Species
Electrophoresis of the amplified product of FUR 1 gene from Candida isolates
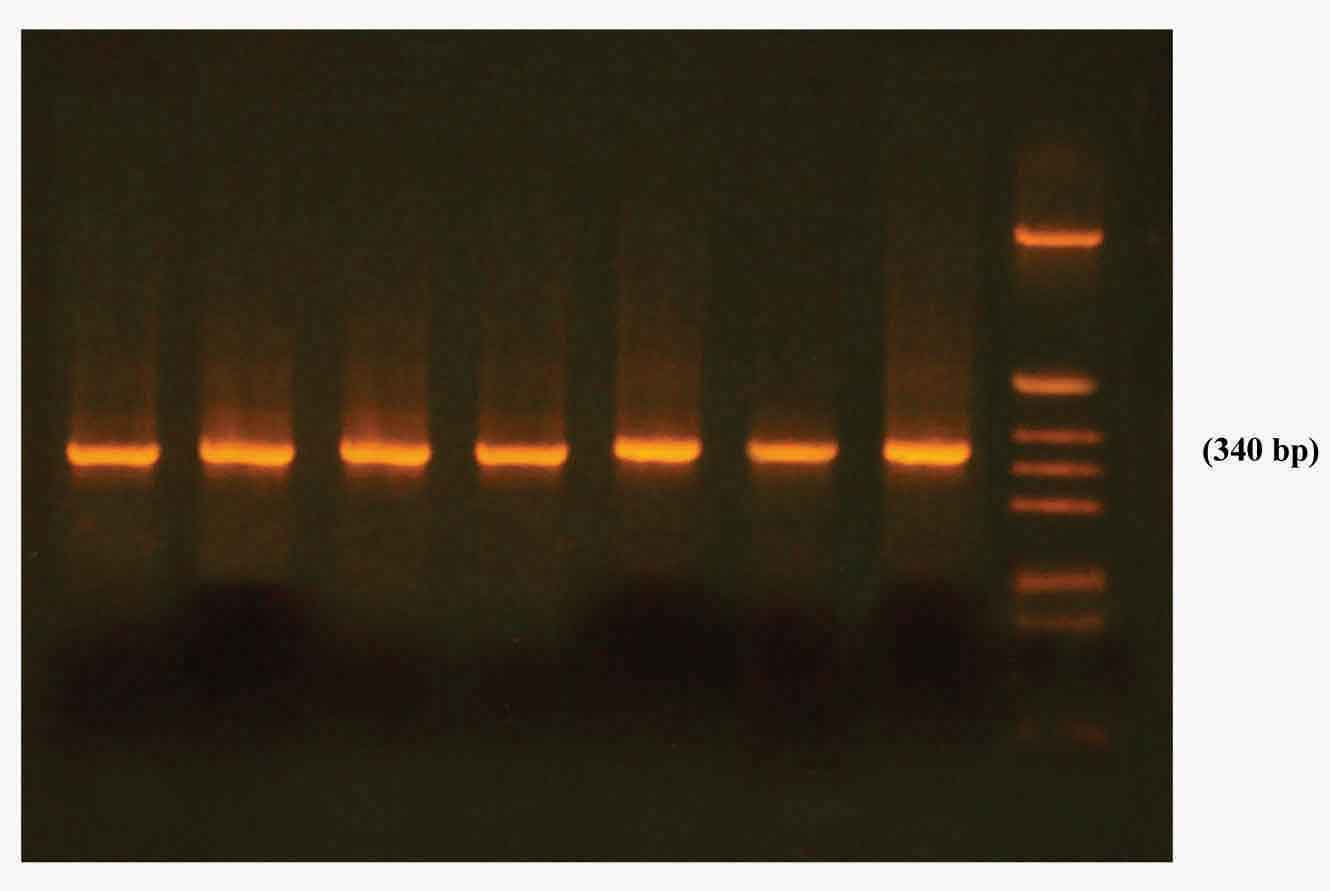
FUR 1 gene was detected in all 5FC resistant isolates.i.e. in 16 C. albicans, 8 C. glabrata, 4 C.krusei and 2 C. tropicalis isolates.
Discussion
Vaginal Candidiasis is a disease of those who are in childbearing ages. The diagnosis of vulvovaginal candidiasis, based solely on signs or symptoms, leads to overestimation of the prevalence of vulvovaginal candidiasis and its overtreatment, while leaving the actual cause of the vaginal symptoms untreated. Hence, for correct diagnosis of vulvovaginal candidiasis, laboratory confirmation of vaginal infection with Candida is necessary. In this study, prevalence of vaginal candidiasis was highest in the age group of 31-40 years (50%), which correlated well with findings of Sobel (50%), Azzam et al., (38.4%) [11] and Jindal et al., (32.03%) [12]. Study subjects in the age group 21-30 years showed second highest prevalence (27.7%). Higher prevalence in these age groups could be because of the influence of sexual activity which peaks during this age [12].
In this study, patients with poor genital hygiene (46.8%) showed higher prevalence of candidiasis than those with satisfactory hygiene (32.67%). Similar findings were also seen in study of Jindal et al. (43.7% and 20.1%) [12]. The present study also showed higher prevalence of candidiasis in users of tight fitting synthetic /nylon underclothes (51.72%). Such underclothes predispose to development of candidiasis due to poor ventilation, leading to increased perineal moisture and temperature.
CHROM agar Candida identified all C.albicans, C.tropicalis, C.glabrata and C. krusei correctly, which correlated with findings of Willinger B. et al., [13] Yucesoy M et al., [14] Momani OM et al., [15] and Gultekin et al., [16]. But in the study of Guelfand L, 100% C.albicans, 92% C. tropicalis, 91% C. krusei were identified and in the study of Houang et al., [17] 100% of C. albicans, C.tropicalis, C. Krusei and 82% of C.glabrata were identified correctly on CHROM agar candida.
In the study of Arzeni et al., [18] varying patterns of antifungal susceptibility were seen. The study of Richter SS et al., showed 3.7%, 3% and 16.2% resistance to fluconazole, 5-flucytosine and itraconazole respectively and study of Sojakova et al., [19] showed 13% resistance to fluconazole and 18.5% resistance to itraconazole. Study of Saporiti et al., [4] showed 13.46% resistance to fluconazole and study of Stiller et al., [5] showed 50% resistance to 5-flucytosine.
In the study of Sojakova, [19] C. glabrata showed 15.2% resistance to fluconazole and 74.1% resistance to itraconazole, which was higher as compared to that in our study and C. krusei showed 41.7% resistance to fluconazole and 58.3% resistance to itraconazole, which was lower as compared to that in our study. C. parapsilosis showed 3.4% resistance to itraconazole, while it showed 50% resistance in the present study. FUR 1 gene detection in 5FC resistant isolates correlated with findings of studies of Dodgson et al.,[6] Hope et al., [20] and Pujol et al., [21].
Resistance among clinical isolates varies greatly worldwide. Modification of the quality or quantity of 14 alpha demethylase is an important mechanism in the development of resistance to azoles. Point mutation in the ERG 11 gene, over expression of 14 alpha demethylase and active efflux of antifungal agents from cells may play important roles in azole resistance. 5FC is a fungicidal drug active against Candida and Cryptococcus spp. 5FC is transported into the cell by a membrane associated permease. Once it is inside the cell, 5FC is deaminated to 5-fluorouracil (5FU) by an enzyme cytosine deaminase encoded by FCY1 gene. Then 5FU is phosphorylated to 5-fluorouridine monophosphate (5FUMP) by uracil phosporibosyltransferase (UPRTase), encoded by FUR1 gene. 5FUMP is then converted into 5-fluorouridine triphosphate, which, when incorporated into RNA in the place of UTP, disrupts protein synthesis. In addition, 5FUMP disrupts DNA synthesis by inhibiting thymidylate synthase. Though Candida spp may show resistance to 5FC through a number of possible mechanisms, resistance to 5FC may result from blocking the formation of 5-fluorouridylic acid (FUMP) by loss of cytosine deaminase activity or by loss of uracil phosphoribosyl transferase (UPRTase) activity. Primary resistance to 5FC occurs due to decreased activity of either cytosine deaminase or UPRTase caused by mutations in the respective genes, FCY1 and FUR1 [6,7]. As studies have shown C.albicans to be the most common species causing VC and as Dodgson AR et al., had shown that 5FC resistance in C.albicans was primarily caused by mutation in position 301 (Cytosine→Thymine) of FUR1, presence of mutant FUR1 gene was looked for among the 5FC resistant Candida spp in the present study. The limitation of this study was that the significant associations with certain epidemiological factors like education, socioeconomic status and religion were not studied and they need to be further investigated in future. Also, women who were being treated for vulvovaginal candidiasis could not be followed up, and so it could not be verified whether treatment was successful. Finally, given the limited duration of the study, a subset of women with recurrent vulvovaginal candidiasis could not be identified, an important condition with epidemiologic features which was distinct from acute vaginal candidiasis [22,23].
Conclusion
Correct diagnosis of vulvovaginal candidiasis and laboratory confirmation of vaginal infection with Candida spp is necessary. C.albicans is the most common species causing vaginal candidiasis in Chennai and among non-albicans Candida spp, C.glabrata is the most common agent. Poor genital hygiene and tight fitting synthetic / nylon underclothes predispose to development of VC in reproductive age group and implicates the need to educate the women regarding genital hygiene and use of well ventilated cotton underclothes. The most reliable test used for speciating Candida is the carbohydrate assimilation test. Azole resistance was low in C. albicans but it was high in non-albicans Candida spp. Prevalence of primary resistance to 5-flucytosine was high in the strains studied and in all of them, it was mediated by mutant FUR1 gene. Continued surveillance and appropriate use of antifungal agents are important, to curb the emergence and spread of antifungal resistance.