Introduction: Calcium is a recognized second messenger implicated in insulin secretion. Vitamin D (1,25-dihydroxycholecalciferol, Calcitriol) plays a role in calcium metabolism. This explains the indirect role of Vitamin D in insulin secretion and insulin sensitivity. Hence, low Vitamin D levels are implicated in decreased insulin secretion and increased insulin resistance. In this study, we tried to find out the probable association of Vitamin D3, calcium and magnesium with reference to insulin resistance in type 2 diabetes mellitus (T2DM) cases. It is well documented that measurement of circulating 25-Hydroxycholecalciferol {25 (OH)Vitamin D3} is a marker of total Vitamin D status.
Methodology: We measured 25(OH) Vitamin D3 levels in thirty T2DM subjects with thirty age and sex matched healthy controls. We estimated Vitamin D status, calcium and magnesium levels in the light of insulin resistance. Insulin resistance was measured by homeostasis model assessment of insulin resistance (HOMA-IR).
Results: Twenty five (OH) Vitamin-D3 level was significantly low among T2DM cases (12.29+2.32ng/ml) in comparison to healthy controls (19.55+0.50ng/ml) (p<0.01). The levels of calcium and magnesium were also significantly low in T2DM cases as compared to healthy controls (p<0.01).
There was significant negative correlation between Vitamin D status and insulin levels, and insulin resistance (p<0.01).
Implication: A significant negative correlation between Vitamin D status and insulin levels suggest that the supplementation of Vitamin D has the potential to increase insulin sensitivity and lower the risk of developing type 2 diabetes mellitus.
Introduction
Vitamin D (1,25-dihydroxycholecalciferol; Calcitriol) is essential for normal growth and development. 7-Dehydrocholesterol is the precursor of Vitamin D which is present in the skin. Upon exposure to UV radiations present in sunlight, cholecalciferol is formed. Cholesterol is converted ultimately to 1,25 Dihydroxycholecalciferol (1,25 DHCC; Calcitriol). 25-Hydroxy cholecalciferol (25(OH) Vitamin D3) formed in the liver reflects total Vitamin D status. Vitamin D is also called as “Sunshine vitamin” [1]. Vitamin D acts like a hormone and its action is mainly on three target tissues namely kidney, intestine and bone. Vitamin D plays an important role in calcium metabolism and in addition, it has a role in cell differentiation, immune regulation and prevention of neoplastic transformation [2]. These variegated effects of Vitamin D are attributed to the Vitamin D receptors present on different types of tissues [3].
Vitamin D deficiency causes growth retardation, muscle weakness and skeletal deformities and rickets in children is an extreme consequence of Vitamin D deficiency [4]. Lesser degree of deficiency or insufficiency may have important implications [5]. Low Vitamin D is indicated with higher rates of increased insulin resistance, weight and BMI (body mass index) [6]. Vitamin D deficiency/insufficiency is a global phenomenon; the deficiency can be found in all age group and ethnicities. While sunlight is an excellent source of Vitamin D, it must be noted that sunscreen, clothing, winter season and skin pigmentation reduces sun exposure of skin to UV rays and ultimately affects Vitamin D synthesis in skin [7, 8]. Vitamin D synthesis requires specific wavelength of sunlight UVB rays (290-310 nm) which is predominantly more during morning hours and in summer days [9]. Evidences are becoming increasingly available in adults suggesting Vitamin D deficiency in adulthood might be associated with chronic disorders including type 2 diabetes mellitus (T2DM). Calcium is important for insulin secretion and Vitamin D has a role in calcium metabolism [10]. This explains the indirect role of Vitamin D in insulin secretion. Certain experimental studies have depicted that Vitamin D is responsible for glucose induced insulin secretion and improve insulin sensitivity, and exerts anti- inflammatory effect [11]. Previous studies have already established an inverse association among Vitamin D status, impaired glucose level and T2DM [12]. Low Vitamin D levels are a risk factor for impaired glucose tolerance and T2DM [13]. However, there are limited studies, which have been performed in the region of Puducherry (Southern India), which reflects the Vitamin D status and insulin resistance observed in T2DM.
In South Indian population, skin pigmentation is more because of direct exposure to sunrays. Skin pigmentation mainly affects the Vitamin D production in skin [8]. So, this study will aid in the understanding of Vitamin D status as a predisposing risk factor to insulin resistance and impaired fasting glucose and ultimately to T2DM in healthy Puducherry population. Data related to association between Vitamin D levels and T2DM, and also with minerals such as calcium and magnesium are also scarce in South Indian population. So, with this background the study was planned to ascertain whether any significant association was present or not between Vitamin D status and serum calcium, magnesium levels, with reference to insulin resistance and as observed in T2DM.
Hence, in the present study, we evaluated the Vitamin D levels in T2DM subjects and normal healthy controls and examined the association of serum Vitamin D, calcium and magnesium levels with markers of insulin resistance namely fasting insulin level and Homeostasis Model Assessment of insulin resistance (HOMA-IR).
Subjects and Methods
This was a case control study that included thirty freshly diagnosed cases of T2DM who had visited the outpatient clinics of General Medicine of Mahatma Gandhi Medical College and Research Institute, Puducherry. The patients were not on any treatment which can affect Vitamin D, magnesium or calcium status. Thirty healthy age and sex matched controls were selected from the same population, but without diabetes mellitus, hypertension, cardiovascular, cerebrovascular disorders, hepatic and renal disorders. Prior to starting this study, ethical approval was obtained from the Institutional Human Ethical Committee (IHEC), Mahatma Gandhi Medical College and Research Institute, Puducherry. Informed written consent was obtained from the participants. Personal, family and dietary history were elicited from the participants by asking standard structured questionnaire.
Subjects with Vitamin D, calcium or magnesium supplementation or those using sunscreen were excluded from the study. Subjects with renal, hepatic or cerebrovascular disorders or endocrinal disorders, females on estrogen therapy, chronic disorders such as tuberculosis, type 1 diabetes mellitus were also excluded from the study. After considering all exclusion and inclusion criteria, thirty T2DM cases and an equal number of healthy controls were selected for this study.
Physiological Parameters of Obesity
Both groups were analyzed for physiological parameters: weight, height, body mass index (BMI), blood pressure (BP), waist circumference (WC), hip circumference (HC) and waist-hip ratio (WHR) to assess general and central obesity.
General obesity: Height was measured to the nearest 0.1 cm, while the subject was standing in an erect position with foot bare on flat floor, against a vertical scale and with heels touching the wall and head straight. The body weight was measured using weighing scale, while the subject was minimally clothed and without shoes, standing motionless on a weighing scale and it was recorded nearest to 0.1 kg.
Body mass index (BMI) was calculated using the formula: BMI=weight (kg)/height(m2).
Central obesity: Waist-Hip ratio (WHR) was calculated to assess central obesity. Waist circumference (in cm) was measured at a point mid-way between the lower rib and iliac crest with the measuring tape centrally positioned at the level of umbilicus. Waist circumference is the average of two measurements, one taken after inspiration and other taken following expiration in standing position. Hip circumference was measured (in cm) at trochanter major of the head of femur.
WHR was calculated using the following formula to assess central obesity: WHR=Waist (cm)/Hip(cm).
Biochemical Analysis of Cardio-Metabolic Risk Factors
Three ml of venous blood sample (fasting) was collected under aseptic precautions from the subjects and controls, following ten hours of overnight fast. Biochemical parameters were analyzed as follows: fasting plasma glucose (analyzed by glucose oxidase and peroxidase method GOD-POD) in Hitachi 902 instrument, plasma insulin fasting (analyzed by automated electrochemiluminiscence), serum triacylglycerols (Glycerol kinase method), 25(OH) Vitamin D (analyzed by CLIA method by automated chemiluminiscence), serum calcium (by Orthocresolpthalein complexone, OCPC method) and alkaline phosphatase (amino antipyrine method). Magnesium in serum was assayed by Calmagite indicator method. All the above mentioned biochemical parameters were estimated using IFCC approved procedures in Hitachi 902 fully automated analyzer and Advia Centaur CP immunoassay system instrument. The internal quality control was undertaken based on QC samples provided by M/s Biorad USA. The external quality assessment was based on the QC sample provided by Clinical Biochemistry Lab, Christian Medical College & Hospital (CMC & H Vellore). As per ADA criteria, T2 DM is confirmed in those having fasting blood glucose above 126mg/dl and postprandial above 200mg/dl, as performed on two different occasions [14]. Hypovitaminosis D is defined as that, wherein the levels of 25(OH) Vitamin D3 are below 25ng/ml. Insulin and 25(OH) D3 were measured by automated chemiluminescence (CLIA) method [6]. Insulin resistance (HOMA-IR) was calculated by using the following formula, where fasting glucose expressed in mmol/l and insulin in mIU/l [15].
HOMA-IR = fasting glucose x Insulin 22.5
The cut-off value to define IR was HOMA-IR ≥ 2.50 [15].
Statistical analysis was done by using SPSS 16.0 software. Test of significance was calculated by unpaired student’s t test between cases and controls. Correlation of Vitamin D with other parameters was performed by two-tailed Pearson’s correlation. Value p<0.05 was statistically significant.
Results
The results of the variables, namely Vitamin D levels, calcium, and magnesium in normal and diabetic cases are shown in [Table/Fig-1]. 25 (OH) Vitamin D3 level was significantly low among T2DM cases than healthy controls (p<0.01). There is hypovitaminosis D among T2DM cases than healthy controls.
Comparison of Vitamin D, Calcium and magnesium levels in T2DM cases
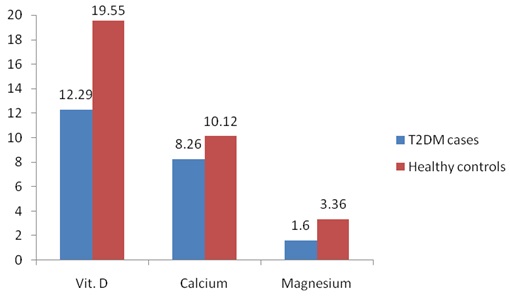
Calcium and magnesium levels were also significantly low in T2DM cases as compared to healthy controls (p<0.01) [Table/Fig-1 and 2]. Similarly, insulin levels and insulin resistance (HOMA-IR) as well as fasting glucose were significantly high among T2DM cases than healthy controls (p<0.01), as shown in [Table/Fig-3].
Comparison of insulin resistance by HOMA-IR in T2DM cases
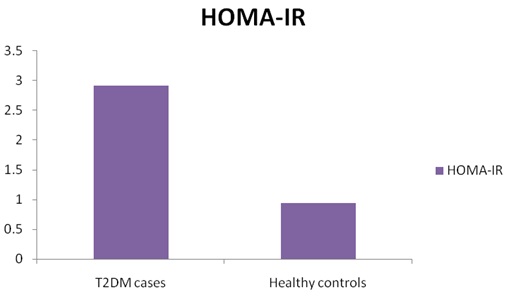
Pearson’s Correlation coefficient of different parameters
| Parameters | Pearson’s correlation coefficient |
|---|
| Serum Calcium with insulin resistance (HOMA-IR) | r= - 0.67** |
| Serum Calcium with serum 25 (OH) Vitamin D3 levels | r= 0.70** |
| Serum Magnesium with serum 25 (OH) Vitamin D3 levels | r= 0.92** |
| Serum Magnesium with insulin resistance (HOMA-IR) | r= - 0.72** |
| serum 25 (OH) Vitamin D3 levels with insulin levels | r= - 0.66** |
| serum 25 (OH) Vitamin D3 levels with insulin resistance (HOMA-IR) | r= - 0.92** |
** P<0.01 (highly siginificant)
There was a negative correlation between Vitamin D and insulin levels (r=-0.66;p<0.01). Vitamin D level bears a negative correlation with insulin resistance as calculated by HOMA-IR (r= -0.92, p<0.01). As Vitamin D level was having negative correlation with insulin resistance, decrease in Vitamin D level is associated with increased insulin resistance. Magnesium level was having negative correlation with insulin resistance as calculated by HOMA-IR (r= -0.72,p<0.01), as shown in [Table/Fig-4]. So, low serum magnesium level is having increased insulin resistance as calculated by HOMA-IR. Magnesium levels was low among T2DM cases in comparison to healthy controls (p<0.01).
25 (OH) D3, Calcium and Magnesium levels among T2DM p value<0.05 is statistically significant.
| Parameters | T2DM cases | Healthy controls |
|---|
| Vit.D level [25 (OH) D3] | 12.29+2.32** | 19.55+0.50 |
| Calcium | 8.26+0.77** | 10.12+0.10 |
| Magnesium | 1.60+0.59** | 3.36+0.15 |
| HOMA-IR | 2.92+0.74** | 0.94+0.10 |
| Glucose fasting | 107.8+20.7** | 86.8+1.07 |
** p value<0.01
Discussion
In our study, Vitamin D level was significantly low among T2DM than healthy controls (p<0.01). There is hypovitaminosis D among T2DM cases than healthy controls. As Vitamin D level was having negative correlation with insulin resistance, so decrease in Vitamin D level is associated with increased in insulin resistance. Vitamin D affects the insulin level as well as insulin sensitivity. The presence of Vitamin D receptors on different tissues explains its diversity of action [4]. Hence, reduced levels of Vitamin D are associated with insulin resistance and may thus be linked to poor glucose control observed in T2DM patients [16,17].
Our results are consistent with previous reports that depicted the fact that hypovitaminosis D in adults may influence the risk of developing diabetes and metabolic syndrome [18,19]. A previous study has shown that Vitamin D levels are positively correlated with insulin sensitivity and negatively correlated with HbA1c levels, indicating Vitamin D deficiency as a predisposing factor in the pathogenesis of diabetes mellitus and cardiovascular diseases [20]. It is believed that future studies will be really helpful to determine the long term adverse effects of Vitamin D deficiency including poor outcome as observed in diabetic management.
In this study, we obtained a significant negative association between Vitamin D level and insulin resistance, independent of other parameters, as obesity and adiposity. We included individuals with only normal BMI (BMI lesser than 30). High BMI and adiposity may affect the levels of Vitamin D by the partly sequestering action enforced by the adipose tissue [21]. Vitamin D is stored in inactive form in liver and adipose tissue. The activation of Vitamin D (hydroxylation reaction) occurs in liver and kidneys and finally gets activated to calcitriol (1,25 DHCC) [22]. In healthy subjects, Vitamin D deficiency occurs mainly because of low dietary intake and less exposure to sunlight. Low or decreased exposure to sunlight (specifically UV-rays in 290-310 nm wavelength), clothing, decreased outdoor activities, and use of sunscreens also affect the Vitamin D status [8]. Seasonal variation also affects the Vitamin D status, mainly in winter [23,24]. Certain other causes such as malabsorption, liver or kidney disorder and obesity also increase the risk for development of Vitamin D deficiency. Obesity is common among T2DM subjects and Vitamin D stored in adipose tissue causes decreased bioavailability [21]. This puts obese T2DM subjects at greater risk of developing Vitamin D deficiency [25]. However, in this study we selected T2DM cases as well as healthy controls with normal BMI.
We also observed low magnesium levels in T2DM subjects, as compared to healthy controls. There was positive correlation of Vitamin D with magnesium levels (r=0.92, p<0.01). According to this finding, Vitamin D levels can also affects the magnesium status [26]. We got negative correlation of magnesium with insulin resistance (r= -0.72, p<0.01). Hypomagnesemia leads to increased insulin resistance (HOMA-IR) [27]. There are already well established facts that low magnesium levels affects glucose metabolism [27,28]. Low magnesium levels are related to obesity [29]. Obesity is associated with increased oxidative stress and insulin resistance [30]. Such a nexus, connecting Vitamin D status in the light of magnesium levels as related to obesity, insulin resistance, and oxidative stress is interesting. Very few reports are available from Southern India and future work needs to be carried out by highlighting the molecular mechanism of Calcitriol action (vis-a-vis) with Vitamin D status (25 hydroxycholecalciferol) [31]. The isolated reports available in the literature, very few talk about calcitriol role in obesity and insulin resistance with reference to magnesium status [30,31]. Magnesium is involved in several facet carbohydrate metabolisms including on insulin release and action [32]. Earlier reports point out that the role of magnesium on insulin resistance is either cause or effect relationship [26, 33]. However, our report clearly depicts that there is hypomagnesemia which is associated with Vitamin D levels in the light of insulin resistance.
In our study, we acquired significant negative correlation between Vitamin D levels and fasting glucose in T2DM subjects. Proper mechanisms linking hypovitaminosis D with increased blood glucose level remains unclear, but there are some supporting studies which indicate that Vitamin D may directly affect pancreatic β-cell secretory functions through nuclear Vitamin D receptors and may affect the insulin sensitivity through insulin receptor expression regulation of intracellular receptors [10–12]. In a few animal studies, administration of calcitriol (activated form of Vitamin D) has been shown to prolong the onset of Type 1 Diabetes mellitus, mainly through immune regulation [19,31]. This is mainly because of effect of Vitamin D on inflammatory pathway [4,19].
Type 2 diabetic patients had reduced levels of Vitamin D than normal individuals. The insulin resistance is more in Vitamin D deficiency. Hence, Vitamin D plays an important role in maintaining normoglycemic condition by influencing insulin secretion. In deficiency state of Vitamin D, there is decreased insulin sensitivity and increased insulin resistance [28]. Chronic Vitamin D deficiency may be a predisposing factor for type 2 diabetes mellitus as per our study. So, in tropical countries like India where there is an abundance of sunlight, still Vitamin D deficiency is quite common. But, Vitamin D deficiency can be avoided by early diagnosis and dietary fortification as well as dietary supplementation of Vitamin D [34]. We also would like to suggest that the inclusion of serum magnesium estimation in obese individual may be made compulsory so that it could be used as a predictor of obesity induced insulin resistance that would eventually predisposes to diabetes mellitus and future micro, and macro vascular complications. We further suggest that magnesium supplementation (diet rich in green leafy vegetables) would help enable the obese individuals to delay the onset of T2DM.
Fortification of foods with Vitamin D can be the proposed treatment for prevention of Vitamin D deficiency [23]. High risk population should be screened thoroughly and must be placed on Vitamin D supplements. Irradiation of milk with UV rays causes enrichment of milk with Vitamin D [34]. Irradiated milk will acts as a rich source of Vitamin D. It may be noted that irradiated milk is a poor source of vitamin A.
As per our observation, we hereby advise all our T2DM patients to take Vitamin D and calcium regularly. Those who are using sunscreen with high SPF (sun protection factor) should receive supplements of Vitamin D and calcium.
Future Perspectives
Vitamin D and calcium as well as magnesium therapy can be initiated during early phase of insulin resistance and T2DM, as it may improve the insulin sensitivity.
But, this requires large prospective cohort study for evaluation of prophylactic and therapeutic roles of Vitamin D and calcium in early phase of insulin resistance and T2DM [18].
Limitations of The Study
Our sample size is small. So, it requires larger sample size to verify the results on a population.
We conducted this study during pre summer months (in March and April). So, we have to conduct this study by keeping in mind the effect of variation in seasons in Vitamin D levels in winter also [1,8].
As skin pigmentation is more in south Indians, it may also affect the levels of Vitamin D.
It may be interesting to carry out further research in ethnic groups who have different degree of skin pigmentation and Vitamin D status.
** P<0.01 (highly siginificant)
** p value<0.01
[1]. Samanek AJ, Croager EJ, Gies P, Milne E, Prince R, McMichael AJ, Estimates of beneficial and harmful sun exposure times during the year for major Australian population centresMed. J. Aust 2006 Apr 3 184(7):338-41. [Google Scholar]
[2]. Szkandera J, Absenger G, Pichler M, Stotz M, Langsenlehner T, Samonigg H, Association of common gene variants in Vitamin D modulating genes and colon cancer recurrenceJ. Cancer Res. Clin. Oncol 2013 Jun 23 [Google Scholar]
[3]. Vinh Qu C L, Ng K, Nguy N LT, The beneficial role of Vitamin D in obesity: possible genetic and cell signaling mechanismsNutr. J 2013 Jun 25 12(1):89 [Google Scholar]
[4]. Nassar MF, Amin DA, Hamed AI, Nassar JF, Abou-Zeid A-EK, Attaby MA, Vitamin D status and scholastic achievement in middle age childhoodJ. Egypt. Soc. Parasitol 2012 Aug 42(2):349-58. [Google Scholar]
[5]. Gradinaru D, Borsa C, Ionescu C, Margina D, Prada GI, Jansen E, Vitamin D status and oxidative stress markers in the elderly with impaired fasting glucose and type 2 diabetes mellitusAging Clin. Exp. Res 2012 Dec 24(6):595-602. [Google Scholar]
[6]. Wright ORL, Hickman IJ, Petchey WG, Sullivan CM, Ong C, Rose FJ, The effect of 25-hydroxyVitamin D on insulin sensitivity in obesity: is it mediated via adiponectin?Can. J. Physiol. Pharmacol 2013 Jun 91(6):496-501. [Google Scholar]
[7]. Engelsen O, The relationship between ultraviolet radiation exposure and Vitamin D statusNutrients 2010 May 2(5):482-95. [Google Scholar]
[8]. Webb AR, Who, what, where and when-influences on cutaneous Vitamin D synthesisProg. Biophys. Mol. Biol 2006 Sep 92(1):17-25. [Google Scholar]
[9]. Guillemant J, Le HT, Maria A, Allemandou A, Pérès G, Guillemant S, Wintertime Vitamin D deficiency in male adolescents: effect on parathyroid function and response to Vitamin D3 supplementsOsteoporos Int 2001 12(10):875-79. [Google Scholar]
[10]. Cangoz S, Chang Y-Y, Chempakaseril SJ, Guduru RC, Huynh LM, John JS, Vitamin D and type 2 diabetes mellitusJ Clin Pharm Ther 2013 Apr 38(2):81-4. [Google Scholar]
[11]. Talaei A, Mohamadi M, Adgi Z, The effect of Vitamin D on insulin resistance in patients with type 2 diabetesDiabetol Metab Syndr 2013 5(1):8 [Google Scholar]
[12]. Bozkurt NC, Cakal E, Sahin M, Ozkaya EC, Firat H, Delibasi T, The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalitiesEndocrine 2012 Jun 41(3):518-25. [Google Scholar]
[13]. Mata-Granados JM, Luque de Castro MD, Quesada Gomez JM, Inappropriate serum levels of retinol, alpha-tocopherol, 25 hydroxyVitamin D3 and 24,25 dihydroxyVitamin D3 levels in healthy Spanish adults: simultaneous assessment by HPLCClin. Biochem 2008 Jun 41(9):676-80. [Google Scholar]
[14]. Sam S, Haffner S, Davidson MH, D’Agostino RB Sr, Feinstein S, Kondos G, Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particle number and size in type 2 diabetesDiabetes 2008 Aug 57(8):2022-7. [Google Scholar]
[15]. Capasso I, Esposito E, Pentimalli F, Montella M, Crispo A, Maurea N, Homeostasis model assessment to detect insulin resistance and identify patients at high risk of breast cancer development: National Cancer Institute of Naples experienceJ. Exp. Clin. Cancer Res 2013 32:14 [Google Scholar]
[16]. Wilmot EG, Edwardson CL, Biddle SJH, Gorely T, Henson J, Khunti K, Prevalence of diabetes and impaired glucose metabolism in younger “at risk” UK adults: insights from the STAND programme of researchDiabet. Med 2013 Jun 30(6):671-5. [Google Scholar]
[17]. Moreno-Pérez O, Portilla J, Escoín C, Alfayate R, Reus S, Merino E, Impact of Vitamin D insufficiency on insulin homeostasis and beta cell function in nondiabetic male HIV- infected patientsHIV Med 2013 May 8 [Google Scholar]
[18]. Tsur A, Feldman BS, Feldhammer I, Hoshen MB, Leibowitz G, Balicer RD, Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetesDiabetes Care 2013 May 36(5):1361-7. [Google Scholar]
[19]. George N, Kumar TP, Antony S, Jayanarayanan S, Paulose CS, Effect of Vitamin D3 in reducing metabolic and oxidative stress in the liver of streptozotocin-induced diabetic ratsBr. J. Nutr 2012 Oct 28 108(8):1410-8. [Google Scholar]
[20]. Ayesha I, Bala TS, Reddy CV, Raghuramulu N, Vitamin D deficiency reduces insulin secretion and turnover in ratsDiabetes Nutr. Metab 2001 Apr 14(2):78-84. [Google Scholar]
[21]. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF, Decreased bioavailability of Vitamin D in obesityAm. J. Clin. Nutr 2000 Sep 72(3):690-3. [Google Scholar]
[22]. Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D, Role of Vitamin D in the pathogenesis of type 2 diabetes mellitusDiabetes Obes Metab 2008 Mar 10(3):185-97. [Google Scholar]
[23]. Davidson MB, Duran P, Lee ML, Friedman TC, High-dose Vitamin D supplementation in people with prediabetes and hypovitaminosis DDiabetes Care 2013 Feb 36(2):260-6. [Google Scholar]
[24]. Diaz S, Vernet M, Paladini A, Fuenzalida H, Deferrari G, Booth CR, Availability of Vitamin D photoconversion weighted UV radiation in southern South AmericaPhotochem. Photobiol. Sci 2011 Dec 10(12):1854-67. [Google Scholar]
[25]. Barengolts E, Vitamin D role and use in prediabetesEndocr Pract 2010 Jun 16(3):476-85. [Google Scholar]
[26]. Niranjan G, Mohanavalli V, Srinivasan AR, Ramesh R, Serum lipid peroxides and magnesium levels following three months of treatment with pioglitazone in patients with Type 2 Diabetes mellitusDiabetes Metab. Syndr 2013 Mar 7(1):35-7. [Google Scholar]
[27]. Paolisso G, Scheen A, D’Onofrio F, Lefèbvre P, Magnesium and glucose homeostasisDiabetologia 1990 Sep 33(9):511-4. [Google Scholar]
[28]. Moreno-Pérez O, Portilla J, Escoín C, Alfayate R, Reus S, Merino E, Impact of Vitamin D insufficiency on insulin homeostasis and beta cell function in nondiabetic male HIV-infected patientsHiv Med2013 May 8 [Google Scholar]
[29]. Chaudhary DP, Sharma R, Bansal DD, Implications of magnesium deficiency in type 2 diabetes: a reviewBiol. Trace Elem. Res 2010 May 134(2):119-29. [Google Scholar]
[30]. Rice BH, Cifelli CJ, Pikosky MA, Miller GD, Dairy components and risk factors for cardiometabolic syndrome: recent evidence and opportunities for future researchAdv. Nutr. Bethesda Md 2011 Sep 2(5):396-407. [Google Scholar]
[31]. Quinn SJ, Thomsen ARB, Egbuna O, Pang J, Baxi K, Goltzman D, CaSR-mediated interactions between calcium and magnesium homeostasis in miceAm. J. Physiol. Endocrinol. Metab 2013 Apr 1 304(7):E724-33. [Google Scholar]
[32]. Rodríguez-Morán M, Guerrero-Romero F, Insulin secretion is decreased in non-diabetic individuals with hypomagnesaemiaDiabetes Metab. Res. Rev 2011 Sep 27(6):590-6. [Google Scholar]
[33]. Guerrero-Romero F, Rodríguez-Morán M, Magnesium improves the beta-cell function to compensate variation of insulin sensitivity: double-blind, randomized clinical trialEur. J. Clin. Invest 2011 Apr 41(4):405-10. [Google Scholar]
[34]. Van Meijl LEC, Vrolix R, Mensink RP, Dairy product consumption and the metabolic syndromeNutr. Res. Rev 2008 Dec 21(2):148-57. [Google Scholar]