For many decades, neuro-scientists have been looking for an ideal stem cell with neural transformation capacities, but they were unable to identify one because of inaccessibility and inability in isolating these cells. In the recent past, olfactory epithelium has been identified as a potential source of such pleuripotent stem cells, which can help in life-long regeneration of olfactory mucosa in adults [1,2]. It was believed earlier that neurogenesis took place only in foetal life. Hippocampus, dentate gyrus, subventricular zone and olfactory epithelium have been shown to have the potential for neurogenesis throughout adult life [3]. The adult Olfactory Epithelium (OE) is a unique and complex tissue containing heterogeneous population of epithelial cells, as it can regenerate itself post injury throughout life. Apart from the support cells and neuroreceptor cells, this complex is said to retain a kind of progenitor cells that are competent for making neurons and non- neural support cells, like olfactory ensheathing cells, oligodendrocytes and Schwann cells [2]. These support cells play a role in the regeneration and myelination processes of normal and injured CNS. Recent studies identified stem like characteristics in a group of cells called Globose Basal Cells (GBCs), residing in the basal part of the olfactory epithelium. Further, a second population of cells called the Horizontal Basal Cells (HBCs) with stem-cell like properties has been described as residing among the GBCs. It therefore appears that the olfactory epithelium contains at least two populations of cells with possible neuropotency [4]. Some studies have shown that there is an increase in the population of Globose stem cells and not the horizontal stem cells during the regenerative process of the olfactory epithelium [5]. Demonstrating GBCs has been extremely difficult in the past, due to absence of specific antibodies and non-standardized isolation procedures [6,7]. There are no definitive markers for GBCs with which the basal cell compartment can be studied. Hence, this research work was conducted, to standardize techniques for isolating these stem cells in pure form by surgical, chemical and immunohistochemical methods.
Material and Methods
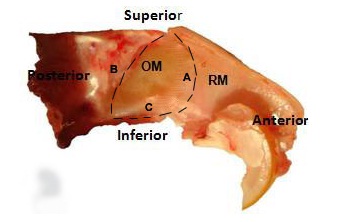
Six Albino Wistar rats were used at different occasions for the purpose of excising the olfactory mucosa from their nasal septa, following all universal precautions. The animals were anaesthetized by intra-peritoneal route using the following combination of drugs: Ketamine – 90 mg/kg body weight and Xylazine – 10 mg/kg body weight. Incision was made over the head region to expose lateral nasal walls. The lower jaw was removed with the help of scissors and a rongeur. The bony walls of the nasal cavity were dissected to expose the bony turbinates. The turbinates were delicately discarded using forceps. The olfactory mucosa was completely excised by standardizing the following three surgical excision lines: the arc of the perpendicular plate, the cribriform plate and the ceiling of the oral cavity [Table/Fig-1]. The olfactory mucosa was immediately placed in frozen DMEM (Dulbeco’s modified Eagle’s medium) culture medium containing antibiotics (Penicillin, Streptomycin, Amphotericin). Olfactory mucosa of rats was also stained with haematoxylin and eosin and it was also compared with the human olfactory mucosa histology. The olfactory mucosa was then treated with 0.5 ml of Dispase II and incubated at 370C for 45 minutes. The olfactory epithelium was separated from the lamina propria using a micro spatula under a Leica EZ2HD dissecting microscope. The olfactory epithelium was then mechanically dissociated and treated with enzymatic cocktail containing Collagenase – 1 mg/ml, Hyaluronidase – 1.5 mg/ml and Trypsin inhibitor – 0.1 mg/ml to separate the olfactory epithelial cells. Cell density was calculated using Neubaeur improved counting chamber and the epithelial cells were plated in the olfactory epithelial culture medium which was standardized, using the following combination of chemicals and reagents: DMEM / F12 (1: 1) – 47.5 ml, 2% FBS – 1.0 ml, N2 supplement – 0.5 ml, EGF (25 ng / ml) – 12.5 μl and L-glutamine– 0.5 ml. Epithelial cells were then immunostained with 10 μl of GBC III antibody and counterstained with 10 μl of R-Phycoerythrin-conjugated Affinipure (Fab’)2 fragment goat anti-mouse IgM which was μ Chain Specific. GBC-III is a mouse monoclonal IgM antibody which recognizes a 40 kDa surface antigen, which is a laminin receptor surface protein. It is highly specific as a marker for GBCs, unlike the earlier antibodies used, like GBC-I, which were nonspecific markers for GBCs and showed positive reactions even with HBCs, sustentacular and duct cells [1]. GBC-III, in powdered form, was reconstituted into the recommended volume of 250 μl. It was used in a concentration of 1:100 for immunostaining. Cells were exposed to the primary antibody for 20 minutes only and to the secondary antibody for 10 minutes. The stained cells were then washed with 1 ml of phosphate buffered solution (PBS) and centrifuged for 5 minutes at 1000 rpm to discard the supernatant. 300 μl of PBS was again added and the suspension was used for isolating pure Globose Basal Cells using Fluorescence-Activated Cell Sorting (FACS). Also, an unstained cell suspension was prepared in PBS, which acted as negative control for FACS.
Nasal septum of rat: OM-olfactory mucosa, RM-respiratory mucosa, A-arc of perpendicular plate, B-cribriform plate, C-ceiling of oral cavity
Results
The basal cells in the olfactory epithelium are believed to be the easily accessible, autologous stem cells in adults. However, selection and isolation of the specific basal cells which are progenitors for neural cells is still being experimented. The present research work helped us to isolate the Globose Basal stem cells in the olfactory epithelium of rats. Surgical excision of the olfactory mucosa along with the nasal septum was done flawlessly by using the following three lines as landmarks- the arc of the perpendicular plate, the cribriform plate, the ceiling of the oral cavity [Table/Fig-1]. Separation technique of the olfactory epithelium from the lamina propria was standardized using the under mentioned procedures-Olfactory mucosa was treated with 0.5 ml of Dispase-II for 45 minutes precisely and fine forceps were used to separate the epithelium from the lamina propria under dissection microscope.
Culture medium used for growing and obtaining maximum confluency of the olfactory epithelial cells was standardized. The epithelial media was composed of chemicals, enzymes and factors essential for epithelial cell proliferation and neurosphere formation. 70% confluency of the epithelial cells was obtained on the 6th day. The culture media was changed every 3 days and the cells were passaged on the 6th day. The passaging of cells was very important for retaining the viable cells and for discarding the dead cells. It further enhanced the capability of the cells for propagating and forming neurospheres. The desirable viable cell density for obtaining optimum growth of epithelial cells on the media was calculated to be 10 X 104. Densities higher than this resulted in overcrowding of cells and loss of cells due to unwanted cell death. Densities lower than this did not provide us with adequate number of cells and hindered neurosphere generation.
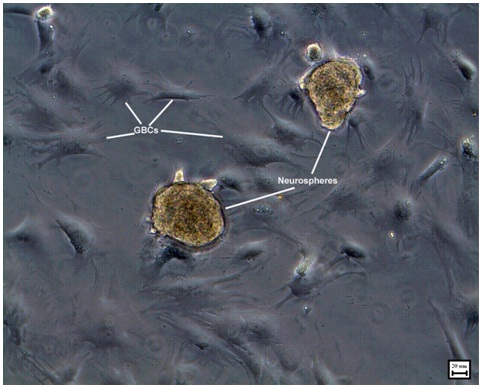
Neurosphere formation began in the olfactory epithelial medium by the end of 3 days. These neurospheres were passaged on the 6th day to obtain the 2nd generation of neurospheres [Table/Fig-2]. Multiple neurospheres were formed by the end of 2 weeks. The self renewal potential of olfactory stem cells was evident from the formation of multiple generations of neurospheres. During this period of 2 weeks, many of the neurospheres had started to proliferate. Hence, an effective method of generating neurospheres was established. Epidermal Growth Factor (EGF) and N-2 supplement seemed to play a significant role in providing an optimum external niche for formation of multiple generations of neurospheres from epithelial stem cells. Epidermal Growth Factor (EGF) is a potential stem cell mitogen allowing cell proliferation through activation of signaling pathway and inducing the production of Beta Fibroblast Growth Factor (bFGF) from the progenitor cells. It was also established that neurosphere generation depended on the initial plating density.
Phase contrast image showing 2nd generation of neurospheres on the 10th day in epithelial medium
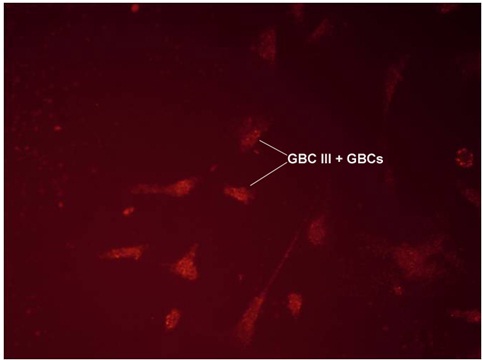
Fluorescence Assisted Cell Sorting (FACS) was used to separate the GBCs from other epithelial cells. GBC-III, a highly specific and sensitive antibody, was used as a marker for GBCs [Table/Fig-3]. This antibody does not react with other cells of the olfactory epithelium or the lamina propria. 99% pure GBCs were obtained using FACS and GBC-III antibody.
Immunofluorescent microscopic picture showing GBC stained with GBC-III antibody
FACS Diva 6.0 software was used to analyze the data using graphs. An area, P1, was chosen with relatively higher percentage of GBC-III stained cells and a graph was plotted, showed the GBC density to be 65.3% of the total number of cells. Another area, P2, a part of P1, with the highest cell density, was chosen and a similar graph was plotted, which showed the GBC density to be 79.9% of the total [Table/Fig-4].
FACS Diva 6.0 graph showing P1 and P2 areas. Green- GBC-III positive cells; Red- GBC-III negative cells
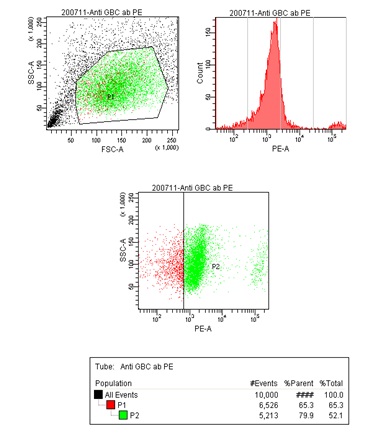
The overall GBC density was calculated to be 52.1%. Total numbers of GBCs sorted were 257,000. The highest number of GBCs were found in the area with the total cell density of 4 x 103. Unstained cell suspension was also run through FACS as a negative control. An insignificant number of 41 positive cells (accounting for 0.4% of the total) was sorted from the negative control, proved the efficacy of the procedure.
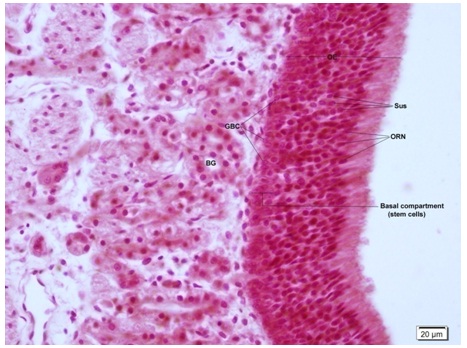
Striking similarities were found during the H and E staining of human and rat olfactory mucosa. The ciliated, pseudostratified OE was made of the similar three basic cell types, namely; Olfactory Receptor Neurons (ORN), basal cells and sustentacular cells. ORNs and sustentacular cells were similar in morphology and location in the OE. The basal compartment of the rat OE consisted of two types of cells, namely: HBCs located closer to the basal lamina and GBCs located in a layer above the HBCs.
The basal compartment of human OE consisted of only one type of cells which were globose in shape which were also called GBCs [Table/Fig-5]. This has been described earlier in the literature and it has been suggested that these human GBCs act and possess the properties of both the cell types present in rodents [8]. The lamina propria was much thicker comparatively in the human OM, with higher density of Bowman’s glands.
H and E staining of human olfactory mucosa (40X). OE-olfactory epithelium, GBC-globose basal cell, Sus-sustetacular cell, ORN-olfactory receptor neuron, BG-bowman’s gland. Basal compartment is seen to contain only globose shaped cells(GBC) and no horizontal basal cells as seen in rat olfactory epithelium
Discussion
Recent studies have identified stem like characteristics in a group of cells called Globose Basal Cells (GBCs), residing in the basal part of the olfactory epithelium. The cells are small, rounded and non-descript cells which form a layer just above the HBCs, which are close to the basal lamina. However, it has been shown by earlier studies [8] and confirmed by this study that only one cell type was present in the basal compartment of humans, which was called GBCs and that they possessed the characteristics of both GBCs and HBCs of rodents. It is extremely important to standardize different procedures involving the isolation and culturing of GBCs universally, to develop conclusive and ideal results in this field. An effective and easy method for excising the OM was developed during this study using the three reference lines as landmarks in the rat nasal septum, as has been described earlier in the text. Separation of OE from the lamina propria has been a hectic task in the past and hence, our work also standardized the composition and duration of the exposure of OM to Dispase II, to easily achieve that. An ideal culture environment and its composition are important for the proliferation and differentiation of the epithelial stem cells. A novel epithelial culture for proliferation of stem cells and neurospheres was developed during this work, which caused minimal changes to the existing composition of the medium, as was suggested by Greco et al., and Xiufang et al., [9,10]. Pure GBCs hence obtained could also be transformed into functional neurons by using different induction media [11]. GBCs had not been identified in the past as the major source of neural progenitor cells in the olfactory epithelium, because specific and sensitive markers for them were not available. They had been identified in the past by negative staining. This study used a highly specific and sensitive monoclonal antibody GBC III to isolate GBCs from other cells in the OE. Earlier studies used non-specific antibodies like GBC I, the expression of which overlapped those of HBCs, Sus, duct cells and neurons [1].
Conclusion
GBCs require an ideal external microenvironment containing both internal and external factors for their proliferation, neurosphere formation and differentiation. Such an environment was formulated and standardized in this study. Isolation of GBCs was standardized, which involved the surgical excision of the olfactory mucosa, separation of the OE from the lamina propria and sorting of these cells using FACS. A highly specific and sensitive antibody (GBC III) for identifying GBCs has been described in the present study. This research work will prove to be beneficial for future research and for understanding the cellular characteristics of GBCs in a significant way.