The main aim of any periodontal intervention is the maintenance of the natural dentition in health and to retain its functional state [1]. Conventional periodontal treatment, such as root planing, gingival curettage and scaling are highly effective at cessation of disease progression and leads to formation of long junctional epithelium which is not desirable since, it is vulnerable for breakdown by bacterial re-colonization. To achieve true regeneration [1] in periodontal defects, regenerative materials like bone graft, Guided Tissue Regenerative (GTR) membranes etc have been tried with varying success rates.
Bone grafting is consider to be as regenerative modality, a material or technique must histologically demonstrate that bone, cementum and a functional periodontal ligament can be formed on a previously diseased root surface. Bone grafts and their synthetic substitutes have been used in an attempt to gain this therapeutic endpoint. Among the graft materials to date, only autogenous bone of extra-oral and intra-oral sources [2–4] is considered as the ‘gold standard’ because it provides the three elements required for bone regeneration – osteogenesis, osteoconduction and osteoinduction [5]. However, autografts have been associated with several shortcomings such as, procurement of enough bone material for recipient site. Alternate substitutes are allografts, alloplasts and xenografts which have been used of late [6,7].
Alloplasts are biodegradable, biocompatible inorganic synthetic grafting material. The degradation rates depend on their chemical composition, structure and physical nature [8]. They are broadly classified as ceramics and polymers. Polymers are further classified as degradable and non degradable [9].
The copolymers of Polylactide (PLA) and Polyglycolic acid (PGA) have already been extensively and successfully used in orthopaedics and cranio-maxillo-facial-for more than a decade.
The other applications of PLA/PGA in dentistry are surgical sutures, absorbable membranes which are used in guided tissue regeneration [10,11]. Recent years absorbable synthetic biopolymers have been used as bone fillers in periodontology, proving effective stimulants to bone regeneration in some cases [12,13] but still they remain controversial regarding there regenerative potential.
In the present study, PLA/PGA is used as regenerative material in treatment of intrabony defect.
Material and Methods
This study is a prospective, randomized, parallel-arm clinical trial which was carried out in the Department of Periodontology and Oral implantology.
The study sample included 30 periodontal intra-bony defects in patients, 15 females and 14 males, aged between 25-50 years who were seeking care for moderate to severe chronic and aggressive periodontitis. They were divided into two groups (test and controls) that consisted of 15 sites each, followed up for a period of 9 months. This study was approved by institutional ethical committee. The inclusion criteria was patients with good general health without any history of systemic disease or compromising medical conditions, clinical and radiographic evidence of periodontal pocket depths more than 5mm. Patients having unacceptable oral hygiene during presurgical phase (phase 1 therapy), gave a history of antibiotics or other medications affecting the periodontium within the previous 6 months, pregnant and lactating females, smokers, third molars, teeth affected by endodontic lesions and/ or inadequate endodontic treatments, overhanging margins or grade III mobility were excluded from study.
Initial therapy (pre-surgical phase) consisted of oral hygiene instructions, thorough full mouth scaling and root planing. Following 6 weeks the patients who showed consistently low level of plaque scores were recruited, randomization was done by using coin toss method. Clinical parameters that is Defect Specific Plaque Score (DSPS) using Turesky et al., modification of Quigley Hein Plaque index, Defect Specific Bleeding Score (DSBS) using Carter and Barnes index, Probing Pocket Depth (PD), Clinical Attachment Level (CAL) and recession (REC) at baseline, six and nine months were recorded by using UNC-15. Radiographs were taken by using paralleling cone technique at the baseline and at the end of study.
Surgical Technique: Surgical procedure was performed as outpatient basis under aseptic conditions. After administering local anesthesia, sulcular incisions were given and full thickness mucoperiosteal flaps were elevated.
Preparation of the site
On surgical exposure 2-wall and 3-wall defect sites were thoroughly scaled and root planed with both hand and ultrasonic instruments. All granulomatous tissue was removed along with very thin portions of bone, which if not adequately vascularized can become necrotic: therefore, the use of curettes, low speed drills and if necessary bone rongeurs of an appropriate size for obtaining the best possible preparation of the receiving site. All the bone cavity, at the end of the treatment, sufficiently had thick borders and without any irregularities.
The test site [Table/Fig-1] was completely filled by using fisiograft (sponge) to the coronal border of the bone defect. The Sponge is cut by means of a scissor or sterile scalpel into fragments with a dimension appropriate for the receiving site. This technique was applied for small pieces, in order to make the graft more malleable it can be hydrated with blood or saline, the fragments were lightly packed by using a cylindrical or ball shaped compactor, until it was completely filled.
Elevation of flap using Kirkland technique and placement of fisiograft mesial to 46 (test site)
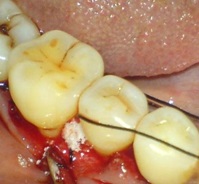
Flaps were replaced and closed by using 3-0 black silk interrupted sutures. Periodontal dressing was given with COE-Pack®. Controls were treated with same technique as mentioned above but without using graft [Table/Fig-2].
Elevation of flap using Kirkland technique showing intrabony defect in relation to 26 (control site)
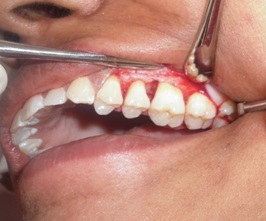
Post-operative care included 0.2% 10ml chlorhexidine gluconate rinse three times daily for a period of 6 weeks. Sutures were removed after two weeks of surgery. Antibiotics amoxicillin 500 mg, metrogyl 400 mg T.I.D for 7 days were prescribed along with analgesic to be used as per required.
Follow up and plaque control was done on the 14th and 30th day respectively. Periodic recall visits were scheduled at 3, 6 and 9 month interval. At these visits, professional oral prophylaxis was done if necessary and oral hygiene instructions were reinforced.
Radiographic assessment
The percentage of bone fill in radiographs was assessed using a software, Corel Draw [Table/Fig-3,4,5and6] with the help of the formula stated below.
IOPA at nine months along with measurements done using corel draw in relation to 46 (test site)
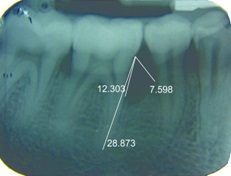
IOPA at baseline showing intrabony defect along with measurements done using corel draw in relation to 46 (test site)
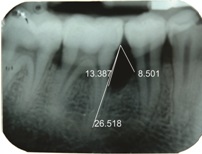
IOPA at baseline showing intrabony defect along with measurements done using corel draw in relation to 26 (control site)
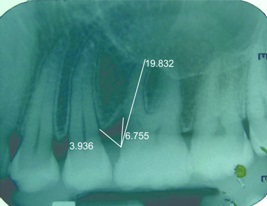
IOPA at nine months along with measurements done using corel draw in relation to 26 (control site)
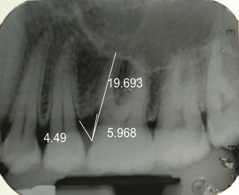
A. Pre-Operative intrabony component (Value A) =

B. Post-Operative intrabony component (Value B) =

C. Amount of bone fill = Value A – Value B

Statistical Analysis
All the data collected was analyzed using SPSS‡ software. Intra-group comparison was done by using ANOVA test#. The independent t test was used to compare the inter-group changes at various time intervals of the study.
Results
The DSPS, DSBS mean score reduction, PD reduction and CAL gain was significant in both the control and test groups [Table/Fig-7,8and9]. On inter-group comparison at baseline, 6 and 9 month the DSPS, DSBS, PD reduction and CAL gain observed no significant differences other than REC seen more in controls compared to tests [Table/Fig-7–9].
Various parameter - control and test group – Base Line
| Group | n | Mean | p |
|---|
| Recession | Control | 15 | 0.467 ± 0.834 | 0.087 |
| Test | 15 | 0.067 ± 0.258 |
| Clinical Attachment level | Control | 15 | 5.667 ± 1.291 | 0.760 |
| Test | 15 | 5.533 ± 1.060 |
| Probing Depth | Control | 15 | 5.267 ± 0.884 | 0.424 |
| Test | 15 | 5.533 ± 0.915 |
| Defect specific bleeding score | Control | 15 | 1.000 ± 0.000 | 1.000 |
| Test | 15 | 1.000 ± 0.378 |
| Defect specific plaque score | Control | 15 | 3.733 ± 0.458 | 0.006 |
| Test | 15 | 3.200 ± 0.414 |
** highly significant at 5 % , n: number of patients, t: is , p: probability value
Various parameter - control and test group – 6 months
| Group | Mean | p |
|---|
| Recession | Control | 0.533 ± 0.915 | 0.032* |
| Test | 0.000 ± 0.000 |
| Clinical Attachment level | Control | 3.867 ±1.457 | 0.238 |
| Test | 3.333 ± 0.900 |
| Probing Depth | Control | 3.400 ± 0.737 | 0.692 |
| Test | 3.533 ±1.060 |
| Defect specific bleeding score | Control | 0.267 ± 0.458 | 0.152 |
| Test | 0.067 ± 0.258 |
| Defect specific plaque score | Control | 1.933 ± 0.458 | 0.310 |
| Test | 1.733 ± 0.594 |
* Significant at 5%
Various parameter - control and test group – 9 months
| Clinical Parameters | Group | Mean | p |
|---|
| Recession | Control | 0.600 ±1.056 | 0.036* |
| Test | 0.000 ± 0.000 |
| Clinical Attachment level | Control | 3.600 ±1.298 | 0.057 |
| Test | 2.800 ± 0.862 |
| Probing Depth | Control | 3.000 ± 0.535 | 0.606 |
| Test | 2.867 ± 0.834 |
| Defect specific bleeding score | Control | 0.067 ± 0.258 | 1.000 |
| Test | 0.067 ± 0.258 |
| Defect specific plaque score | Control | 1.333 ± 0.488 | 0.526 |
| Test | 1.467 ±0.640 |
* Significant at 5%
Amount of defect fill
The amount of defect fill in both groups from baseline to 9 months was statistically significant (p<0.05). The percentage defect fill at nine months post-surgery was 36.933 ± 24.697 and 46.800 ± 30.086 in control and test group respectively. But on comparing the two groups there was no statistical difference in mean percentage defect fill [Table/Fig-10].
Mean % of bone fill in control and test group at nine Months
| Mean | p |
|---|
| Control Group | 36.933 ± 24.697 | 0.335 |
| Test Group | 46.800 ± 30.086 |
Discussion
On considering various bone grafting materials used in the treatment of intrabony defects, biomaterials/bone substitutes have been used with varying success rates to accomplish the re-construction of the lost periodontal attachment apparatus. Resorbable synthetic polymers have been developed by the biomedical research over the last decades among them PLA /PGA has been used as an osteoconductive material both medical and dental fields.
It is a low density copolymer of polylactide-polyglycolide, displays a good handling properties during the surgery; degradation occurs through “bulk erosion” by hydrolysis in a period ranging from 3 to 6 months, depending on the host factors, location of implanted material and degree of circulation of the area. The small mass and large surface area of Fisiograft® ‡ permit fibroblasts to easily penetrate and initiate its absorption and cell colonization of the material that is implanted. In addition the spongy structure does not provide any hindrance to the advancing osteocytes, there bone formation and mineralization.
The present study aims at evaluation of the regenerative potential of the Fisiograft® in treating intrabony periodontal defects. So far, to the author’s knowledge three clinical trials [14–16] have been done using various forms of Fisiograft® in intrabony defects showing contradictory results.
On comparison, our study results correlate with the Minenna et al., study [14] which showed no significant changes in clinical parameters, CAL gain and PD reduction among the groups at the end of the study period, however gingival margin position showed statistically significant apical shift in controls, whereas Minnena reported no significant differences in recession depth among groups [Table/Fig-7–9]. The study results are in contrast with studies of Stratul et al., [15] and Bansal et al., [16].
On radiographic examination the amount of defect fill in controls and tests showed no significant changes at 9 months which is in accordance with Minnena et al., [14] and contrast to Straul et al., [15] and Bansal et al., study [16] [Table/Fig-10].
Bertolli et al., [17] compared fisiograft with autogenous graft and Platelet Rich Plasma (PRP) in the treatment of human osseous defects and fisiograft showed statistically significant results over 6 months but promotes delayed bone healing.
On further comparing the results with similar studies by using polymers Yukna et al., [18] showed the use of HTR in treatment of intrabony defects gave superior results over OFD alone. The use of HTR polymer once again proved to be an effective regenerative material in Prakash et al., study[19]showing significant results over OFD alone.
In a comparative study by Meadows [20], the use of Polylactic Acid (PLA) in intrabony defects did not prove effective over OFD along with Decalcified freeze dried bone allograft and OFD alone.
Systematic review on graft materials and their biological agents by Trombelli et al., [21]. PLA granules when used as intrabony defects showed no significant results over other graft biomaterials in CAL gain and PPD reduction when compared to OFD procedure.
Conclusion
The synthetic copolymer used in this study did not prove to be efficacious regenerative material. The clinical and radiological parameters on intergroup comparison did not give any statistically significant results however, gingival margin showed greater apical shift in controls.
The material was biocompatible as it did not show any inflammatory changes in any subject of the test group. Overall, the results in this study indicated a limited adjunctive effect of biodegradable polymer in periodontal reconstruction procedure.
The limitations of the study are small sample size and to add more authenticity to the study, histological examination would have been done to evaluate regeneration in both groups.
‡Statistical Package for the Social Sciences
#Analysis of Variance
‡Ghimass RI, Casalecchio dI, Italy
** highly significant at 5 % , n: number of patients, t: is , p: probability value
* Significant at 5%
* Significant at 5%