The primary etiological factor for periodontal diseases is dental plaque, which results in progression of inflammation to the supporting tissues. Removal of the cause (and its effects) is the primary goal of both non-surgical and surgical treatment. The major non-surgical therapeutic approach involves mechanical Scaling and Root Planing (SRP). The infective nature of the disease has lead to the widespread use of antimicrobials, as an adjunct to SRP. There are many antibiotics available which are released in the simulated gingival fluid for about 5 days [1]. Tetracycline is one of them with the highest level of drug being released during 24 hour broad-spectrum antibiotic that inhibits the anaerobic and followed by sustained effect up to 5 days [2]. Goodson et al., was the first to study local drug delivery system for periodontal diseases using ethylene vinyl acetate fibers incorporated with tetracycline hydrochloride, which exhibited in vitro drug release up to 9 days [3]. Afterwards, other researchers attempted to develop controlled release devices using various polymers and antibiotics and evaluated in vitro or in vivo for the treatment of periodontal diseases. Doxycycline is the most common broad spectrum antibiotic and is active against most of the periodontal pathogens due to the low minimum inhibitory concentration (MIC) [4]. Polye-caprolactone (PCL) is a semi-crystalline biodegradable aliphatic polyester which is well known for its slow biodegradability, high biocompatibility and good drug permeability [5]. Unlike commonly used biodegradable polymers such as poly (D,L-lactic-co-glycolic acid), PCL does not produce a local acidic environment as it degrades [6]. This, along with its comparatively low cost, renders PCL an attractive biomedical polymer. However, PCL, in the form of homopolymer, has not been used for periodontal diseases successfully yet. It has been tested invitro as a matrix in the form of fiber for tetracycline hydrochloride delivery and as a film for minocycline delivery in periodontal therapy. The main objective of the present study was to develop a local delivery system in the form of Doxycycline electrospun PCL nanofibers for treatment of periodontal infections.
Material and Methods
Doxycycline (99.90% purity) was received as gift samples from Nandlal and Bankatlal Mumbai, India. Polycaprolactone (PCL) was from Sigma aldrich Chemicals, Mumbai, India. All the chemicals used were of analytical grade.
The key ingredients used in formulation are listed below with their function in the formulation:
DOX: API (Active Pharmaceutical Ingredient).
Polycaprolactone: Drug carrier, fiber formation & release retardation.
Method
Solution of PCL with DOX was prepared, and the nanofibers were prepared by electrospinning technique in Department of Pharmaceutics, IIT, BHU, Varanasi (UP), India. Scanning electron microscopy (SEM) was used to investigate the morphology and average diameter of the electrospun nanofibers. Electrospinning is an inexpensive method that creates polymeric fibers with diameters in the range of nano to a few microns through electrically charged jet of polymer solution or polymer melt. When charges within a polymer droplet at the tip of a needle reach a critical amount, a fluid jet will erupt from the droplet. The electrospinning jet will travel towards a grounded collector. As the solvent evaporates, the jet solidifies and the polymeric fibers collect on the grounded target [7]. Drugs can be capsulated directly into electrospun fibers by electrospinning of a mixture solution of a drug and polymer. The solubility and compatibility of drugs in the drug–polymer–solvent system are the effective factors on drug release behavior although both hydrophobic and hydrophilic drugs can be incorporated in electrospun fibers [8,9]. Electrospun nanofibers offer advantages such as higher drug loading efficiency in comparison with some other methods like encapsulation [10]. Furthermore, the drug release profile can be tailored by a modulation on the morphology, porosity and composition of nanofibers [11]. Very small diameter of nanofibers can provide a short diffusion passage length and their high surface area is helpful to mass transfer and efficient drug release [12].
Electrospinning was carried out using 13% w/v solution of PCL in methanol and chloroform mixtures with ratios of 3.5:1.5 v/v. Then drug was added which is soluble in polymer solution. The drug concentration was in the range of 25– 35% w/w with respect to the polymer used. The resulted clear solution was transferred to a 5-ml syringe pump with a right angle-shaped needle of 0.6 mm in inner diameter attached to it. The flow rate of the polymer solution was 1.50–2.50 ml/h, and the applied positive voltage was in the range of 12–14 kV. The resulting fibers were collected on a grounded aluminum plate. The distance between the needle tip and the grounded target was 15 cm. The thickness of all nanofibers webs ranged from 300 to 340 μm. For preparation of electrospun nanofiber horizontal set up was used.
The uniformity of thickness was determined by measuring thickness of selected nanofiber (surface area 1cm) of each formulation using a screw gauge. The uniformity of weight was determined by weighing each nanofiber film (surface area 1cm) of each formulation on electronic balance. The drug loaded nanofiber film surface area 1cm were cut and kept in 100 ml mcilvaine buffer of pH(6.6) by vigorous stirring for 6 hours in a tightly closed conical flask. The amount of drug present was measured spectrophotometrically.
Scanning Electron Microscopy Studies (SEM) [
Table/Fig-2]
Surface morphology of nanofiber was studied by SEM. The mounted samples were sputter coated for 5 min with gold using fine coat ion sputter and examined under SEM. The SEM photographs demonstrated that no beaded fibers were obtained by electrospinning of these solutions containing various amounts of DOX. This study included forty bleeding sites, with a probing depth 5-8mm, which were selected in 7 patients of both genders (4 females and 3 males) aged between 20 to 50 years from the outpatient Department at Faculty of Dental Sciences, IMS, BHU, Varanasi, Uttar Pradesh, India. The Ethical committee of Institute of Medical Sciences, BHU, Varanasi, Uttar Pradesh, India, approved the study and written informed consent was obtained from all patients. Patients with good systemic health, patients who had not received any surgical or non-surgical periodontal therapy in the past 6 months, who were diagnosed as suffering from chronic generalized periodontitis, were enrolled. Individuals with history of using anti-microbial mouthrinses within 2 months of the baseline visit or on routine basis or patients having a history of allergy to doxycycline or lidocaine, were excluded from the study.
The selected sites were randomly divided into 2 groups:
Scanning Electron Microscope photograph.

Test Group A (SRP + DOX nanofibers) – Included 20 sites treated by SRP(Scaling and Root Planing) with Doxycycline nanofibers.
Test Group B (SRP alone) -Included 20 sites treated with SRP alone.
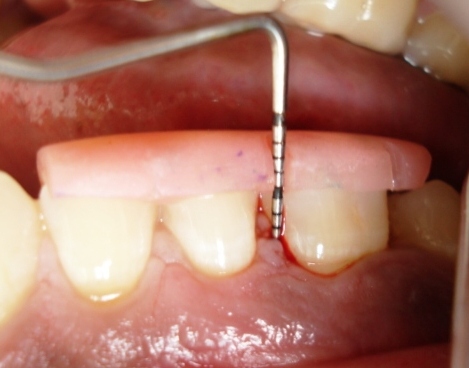
The clinical parameters recorded were the probing depth (PD) measured with UNC-15 periodontal probe using customized acrylic stent [Table/Fig-3], plaque index (PI) and gingival index (GI). After recording clinical parameters from each site at baseline, a thorough SRP was done, in both the groups. The clinical parameters were assessed at baseline, after 15 days and 1 month after receiving treatment in a same patient; as it is a split-mouth study.
Pre-operative probing depth at baseline.
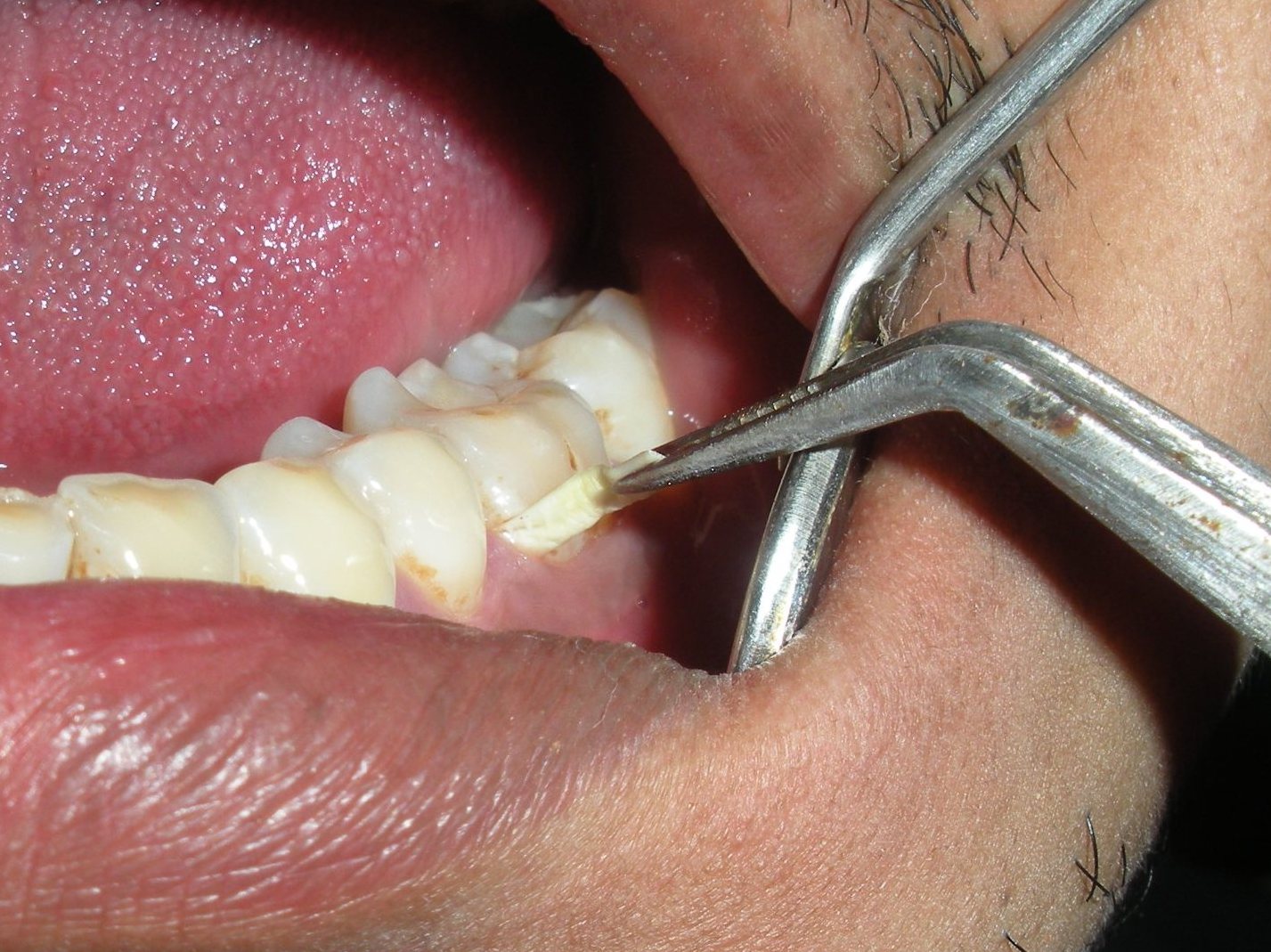
Administration of nanofibers was accomplished by isolating the selected site with cotton rolls and then inserting the fibers subgingivally directly into the base of the pocket. Gentle force was used with straight probe, so that the material fills the depths and curves of the pocket. The gingiva was subsequently and carefully adapted to close the entrance of the gingival margin and hand pressure was applied for just a few minutes to encourage hemostasis and initial setting of the material inside the pocket. The gingival margin was sealed with Coepak to prevent the dislodgement of the drug and to prevent the ingress of oral fluids. As degradation of PCL is slow in aqueous medium due to its semi-crystalline and hydrophobic nature, the drug release from PCL electrospun nanofibers over these periods of time was controlled by diffusion mechanism. Patients were recalled after 7 days for Coe-pak removal and were evaluated for any inflammatory response. Patients were instructed not to chew any hard, crunchy or sticky food for at least 1 week, postpone brushing and flossing on the treated site for 1 week, not to disturb the area with tongue, finger or tooth pick, and to report immediately if the material is dislodged before the scheduled recall visit or if pain, swelling or any other problem occurs.
Insertion of the nanofiber into the periodontal pocket.
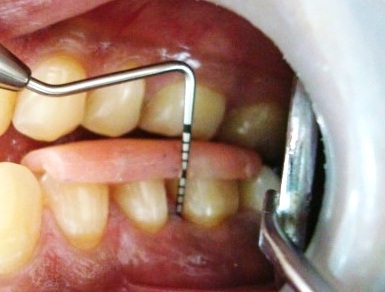
Post-operative probing depth after 1 month.
Results
The recording of all the clinical parameters were done at baseline, after 15 days and 30 days. Finally, the complete data was statistically analyzed using paired t–test. None of the subjects reported any oral symptoms such as toothache (including dental or gingival), painful symptomatology (including oral pain, tenderness, soreness, discomfort or sensitivity), inflammation, allergy, abscess, altered taste or increased salivation, etc.
Comparison of two groups
Group A treated with SRP (Scaling and Root Planing) + DOX and Group B treated with SRP alone. At the end of the observation interval (i.e. 15th and 30th day), all sites got healed unevenly. The mean value for PD, PI and GI score was measured at 15th and 30th day following treatment. The percentage reduction in PD, PI and GI score from base line were 68%, 85% and 90% respectively, in a patient treated with Group A (SRP+DOX), whereas in the patient treated with Group B (SRP alone) from the base line were about 50%, 71%, and 71% respectively, which shows that the formulation containing DOX was significantly better.
In-Vitro Release Studies of DOX
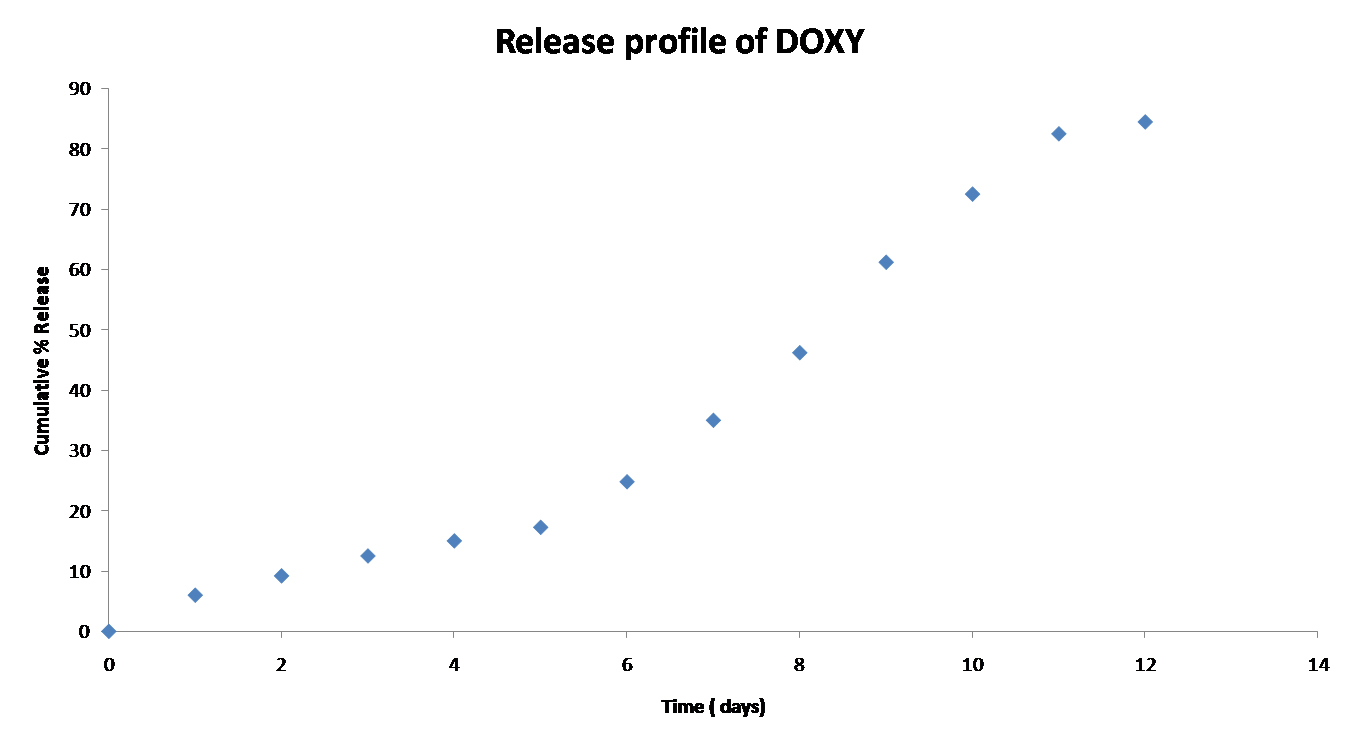
The release profile of DOX shows sustained release i.e. 30 to 40% of drug release occurs in 6 days [Table/Fig-6]. Later part of the profile shows burst release this may be due to loosening of nanofiber and easy penetration of dissolution medium inside the nanofibers. To study the release kinetics of DOX from nanofiber, regression coefficient value (R2) was calculated using different models. A zero order equation showed better fit than first order and Higuchi equation shown in [Table/Fig-7 and 8] (0.938). In order to understand the release mechanism of DOX from electrospun nanofiber, Peppas equation were used and the ‘n’ value found to be 1.158, which indicates zero order release by erosion.
In-vitro Release of DOX formulation.
Comparison of Group A with Group B
| | 15th Day | | | 30th Day | | | |
|---|
| Parameter | Base line mean (SD) | Mean (SD) | Change from baseline | p | Mean (SD) | Change from baseline | p | %reduction |
|---|
| Probing Depth |
| Group (B) SRP | 6.85±1.18 (100%) | 5±0.86 (73%) | 1.8 | <0.01 | 3.4±1.10 (50%) | 3.4 | <0.01 | 50 |
| SRP + Dox (A) | 6.5±1.05 (100%) | 4.1±1.02 (63%) | 2.4 | <0.01 | 2.1±0.97 (32%) | 4.4 | <0.01 | 68 |
| Plaque Index |
| SRP (B) | 2.4± 0.31 (100%) | 1.15± 0.33 (47%) | 1.25 | <0.01 | 0.7±0.47 (29%) | 1.7 | <0.01 | 71 |
| SRP + Dox (A) | 2.263± 0.42 (100%) | 0.88±.28 (38%) | 1.38 | <0.01 | 0.34± 0.31 (15%) | 1.92 | <0.01 | 85 |
| Gingival Index |
| SRP (B) | 2.63±.28 (100%) | 1.31±.038 (49%) | 1.32 | <0.01 | 0.78±0.45 (29%) | 1.85 | <0.01 | 71 |
| SRP + Dox (A) | 2.51±0.18 (100%) | 0.63±0.30 (25%) | 1.88 | <0.01 | 0.26±0.24 (10%) | 2.25 | <0.01 | 90 |
Paired “t” test: p < 0.05 is significant. Group A: treatment mode SRP +DOX. Group B: treatment mode SRP. Clinical parameters showed significant results in Group A (SRP +DOX). In Group A-Probing depth reduction, Plaque Index and Gingival index reduction was 68%, 85% and 90% respectively, which was significant than Group B- Probing depth reduction, Plaque Index and Gingival index reduction was 50%, 71% and 71% respectively.
Kinetics Modeling of DOX Fiber
| Drug | Zero order | First order | Higuchi |
|---|
| K(Mg/time) | R2 | K time-1 | R2 | K Mg/√time | R2 |
|---|
| DOX | 7.5207 | 0.9385 | 0.0673 | 0.8476 | 26.341 | 0.7693 |
Discussion
Local delivery of antimicrobial agents is becoming more prevalent since it leads to higher concentration of the drug at the intended site of action using a lower dose with an associated reduction in side effects relative to systemic administration. Local route of drug delivery provides direct access to the systemic circulation through the jugular vein bypassing the first pass hepatic metabolism leading to high bioavailability [13]. Other advantages include excellent accessibility, low enzymatic activity, painless administration. In the present study, an attempt was made to evaluate effectiveness of doxycycline nanofibers in the treatment of chronic periodontitis, as an adjunct to SRP (Scaling and Root Planing). Doxycycline was chosen in the present study, because of its proven efficacy in the management of periodontal diseases. Doxycycline is known for its antibacterial actions and is a bacteriostatic agent that inhibits bacterial protein synthesis [14]. Other additional properties include collagenase inhibition, anti-inflammatory actions and inhibition of bone resorption. It however, has the ability to bind to the hard tissue walls of pockets to establish a drug reservoir. The results from the present study suggest that the application of doxycycline nanofibers combined with SRP (Scaling and Root Planing) is beneficial in the treatment of chronic periodontitis and improving periodontal parameters for 1 month duration. The drug concentration and a sustained release of drugs were achieved from the nanofibers for at least 19 days with low burst release. This could be an ideal treatment period for periodontal diseases. All electrospun nanofibers remained smooth and quite flexible, without shrinkage during the period of our treatments, which may offer a desirable texture to be used comfortably. In order to be effective, the drug should reach the entire periodontal pocket up to the bottom and should be maintained long enough at a sufficient concentration for the intended pharmaceutical effect to occur [15]. Periodontal pocket have a complicated anatomic characteristics and also periodontal pathogens in subgingival environment reside in a biofilm adhering to the exposed root cementum or even invading the pocket epithelium [16]. Thus, high concentrations of antimicrobial agents are needed before a beneficial effect can be expected. Gingival crevicular fluid (GCF) concentrations to peak with a concentration of 1473-1986 mg/ml at 2 hours and gradually decreased to 309 mg/ml at the end of 7 days which was above the minimum inhibiting concentration for doxycycline [17]. Several studies comparing SRP with doxycycline local gels have revealed similar results, which indicates either SRP or doxycycline gel alone may be favorable [18]. However, as this material i.e. doxycycline nanofibers are easy to place in periodontal pocket, less time consuming and is relatively cost effective. As with any other research this study also has some limitations like adverse reactions with these nanoparticles or drug incorporated in it may impose risks to the patient.
Conclusion and Future Perspective
In the futuristic ideal, these nanofibers would be used as feasible and attractive alternative for non-invasive, precise, timed and targeted delivery of drugs. These PCL electrospun nanofibers can be used as a locally controlled delivery system and the doxycycline nanofibers had more additive benefits when used in adjunct to scaling and root planing in patients with chronic periodontitis.