Medicinal plants have been used since ancient times by humans for the treatment and prevention of various systemic diseases. The rural population of India and various other Asian countries is hugely dependent on these plants for the treatment of diseases. They are especially preferred because of their minimal side-effects. These ancient practices are supported by appropriate scientific background [1,2]. In fact, the proven effectiveness against many diseases have given herbal products a pre-eminent place in the culture of south Asia. Even today, many of these medicinal plants are being used for oral health care and treatment of common periodontal diseases.
Spirulina is a blue-green algae that is associated with a wide range of nutritional and health benefits. Phycocyanin (Pc) is one of the major pigment constituents of Spirulina, a microalgae used in many countries as dietary supplement, whose nutritional and therapeutic values have been very well documented [3–5]. Phycocyanin exists as a complex interacting mixture of trimer, hexamer and decamer aggregates. The relative amount of each species has been reported to be a function of pH, ionic strength, temperature and protein concentration [6]. At present, there is a mass of evidence in favour of the antioxidant properties of Phycocyanin, which have been used to explain, at least in part, its anti-inflammatory effects. It is well known that Reactive Oxygen Species (ROS) are involved in a diversity of important pathological processes in medicine, including among others: inflammatory and neurodegenerative diseases, atherosclerosis, cancer and reperfusion injuries. The researches done in the past have shown the potential benefits of regular spirulina intake in the prevention of many pathological disorders associated with oxidative stress and inflammation. Periodontitis is an inflammatory disease induced by bacterial biofilms that accumulate in the gingival margin, in which a series of inflammatory responses are initiated in periodontal tissues by endogenous gram negative periodontal bacteria [7]. Little research has so far been done on the effect of spirulina on the periodontal health. Hence, this study is first of this kind, which has assessed the clinical effect of spirulina gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis.
Material and Methods
Forty subjects were recruited from the patient pool of the Department of Periodontology, Meenakshi Ammal Dental College, Chennai, India with approval from ethical committee and by following the Declarations of Helsinki [8]. Consents were obtained from the patients. Sixty four sites in the mandibular anterior region with minimum probing pocket depths of ≥5mm were selected in both male and female subjects, aged 25 to 45 years, who were diagnosed with chronic periodontitis. The inclusion criteria included systemically healthy patients with generalized chronic periodontitis, with Probing Pocket Depths (PPDs) ≥5mm and those who had the ability to maintain optimum oral hygiene, after the initial phase of treatment. A stent was prepared and probing pocket depth was measured before and after the treatment. The stent enabled the periodontal probe to reach the same position before and after the treatment, to avoid error or bias during treatment. Patients with aggressive periodontitis, pregnant females, smokers and those with history of antibiotic or periodontal therapy in preceding 6 months, were excluded from this study. The subjects were randomized and selected, based on coin toss method and they were categorized into two treatment groups. All the patients participated in the study, with no drop out cases.
Group A (Experimental): Thirty three sites (in the mandibular anterior region) were treated with Scaling and Root Planing (SRP) and sub–gingival application of spirulina gel done, which was used as local drug delivery.
Group B (Control): Thirty one (mandibular anterior region) were treated with SRP alone.
All the subjects selected in this study received supragingival scaling and were given oral hygiene instruction prior to the commencement of the study. Customized stents were prepared for the sites where the gel was to be administered for standardization of the parameters throughout the course of the study. The clinical parameters such as Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL) were assessed at baseline (120th day), before SRP and local delivery of spirulina gel in Group A and prior to SRP alone in Group B and then again at 120th day after the treatment protocol.
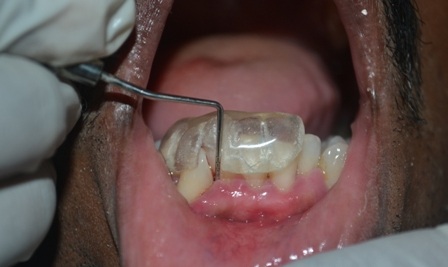
[Table/Fig-1].
Pre–Operative Photograph; Probing Pocket Depth ≥5 mm
Preparation of Spirulina Gel
In this study, pure extract of spirulina algae powder was obtained and an aqueous based gel was prepared. The constituents of spirulina containing gel were prepared in the following proportion: Spirulina powder (4gm), Gelatin-12gm, Glycerin (wetting agent) 0.2ml, Peppermint oil (flavouring agent)-0.1 ml, Sodium saccharine (sweetening agent)-0.1ml and Purified water q.s100ml. All the ingredients were homogenized using a homogenizer, to obtain the gel. All the ingredients of the gel served as an inert vehicle for the drug.
Local Drug Delivery
Thorough scaling and root planing was done for both the groups and the areas were properly irrigated with physiologic saline. For standardization, spirulina gel was injected into the periodontal pockets in Group A by using a syringe with a blunt cannula. No periodontal dressing was applied after the delivery of the drug. After placement of the in situ gel, patients were instructed to refrain from chewing hard or sticky foods, brushing near the treated areas, or using any interdental aids for 1 week. Adverse effects were noted at recall visits, and any existent supra–gingival deposits were removed.
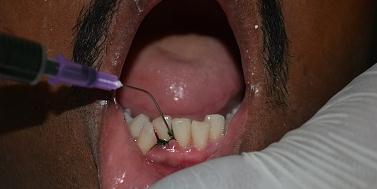
[Table/Fig-2].
Application of Spirulina Gel
Statistical Analysis
Student’s paired t-test was employed to find the significance of mean of PPD and CAL individually for experimental and control groups between 0-day (baseline) and 120th day after the treatment protocol.
Analysis of Covariance test was applied to compare the post treatment values of experimental and control groups on 120th day after adjusting with the baseline scores of the two groups.
For all the tests, a p-value of 0.05 or less was considered as a level of significance.
Results
1. Comparison between the mean Probing Pocket Depth (PPM) and Clinical Attachment Level (CAL) at Baseline (mean – SD) and on 120th day separately for Experimental and Control groups:
In the experimental group (Group A- SRP + Local Drug Delivery with spirulina), t– test was applied to compare mean PPD and CAL at 0th day (baseline) and 120th day (post treatment). It was found that at baseline, the mean PPD was 3.36 mm and CAL level was 4.52 mm, which reduced to 1.79 mm and 3.39 mm respectively [Table/Fig-3]. This was statistically significant (p ≤0.05).
Comparison between the PPD and CAL at Baseline (mean – SD) and 120th day separately for Experimental and Control groups Level of significance p value ≤ 0.05
| Group | No. of sites | 0th day (baseline) | 120th day (post–treatment) | Paired t–test value | p- value |
|---|
| Mean | SD | Mean | SD |
|---|
| PPD |
| Experimental | 33 | 3.36 | 0.603 | 1.79 | 0.485 | 14.745 | 0.001 |
| Control | 31 | 3.45 | 0.961 | 2.61 | 0.844 | 5.996 | 0.001 |
| CAL |
| Experimental | 33 | 4.52 | 1.064 | 3.39 | 1.088 | 6.707 | 0.001 |
| Control | 31 | 4.52 | 0.851 | 3.84 | 1.003 | 5.044 | 0.001 |
In the control group (Groups B-SRP alone), t-test was applied to compare mean PPD and CAL on 0 day (baseline) and on 120th day (post treatment). It was found that at baseline, the mean PPD was 3.45 mm and CAL level was 4.52 mm, which reduced to 2.61 mm and 3.84 mm respectively [Table/Fig-3]. This was statistically significant p ≤0.05).
2. Comparison of Mean PPD and CAL on 120th day (post treatment) between the experimental and control groups after adjusting with the baseline values.
The Co-variance test was applied to compare the post–treatment mean scores of PPD and CAL between experimental (Group A) and control groups (Group B) after adjusting with the baseline values of the 2 groups. As has been seen in [Table/Fig-4], the p–value was significant, which inferred that post treatment scores of experimental and control groups were statistically different. This conferred that the mean PPD had decreased in the experimental Group A (SRP + Spirulina). Also, there was gain in CAL as compared to that in the control Group B (SRP alone).
Comparison of Mean PPD and CAL at 120th day (post treatment) between the experimental and control groups after adjusting with the baseline value Level of significance p value ≤ 0.05.
| Group | No. of sites | 0th day (baseline) | 120thday (post–treatment) | ANCOVA-test result ‘f” –Value | p- value |
|---|
| Mean | SD | Mean | SD |
|---|
| PPM |
| Experimental | 33 | 3.36 | 0.603 | 1.79 | 0.485 | Group=30.185 | 0.001 |
| Control | 31 | 3.45 | 0.961 | 2.61 | 0.844 | Baseline value=28.29 | 0.001 |
| CAL |
| Experimental | 33 | 4.52 | 1.064 | 3.39 | 1.088 | Group=4.732 | 0.001 |
| Control | 31 | 4.52 | 0.851 | 3.84 | 1.003 | Baseline value=41.178 | 0.001 |
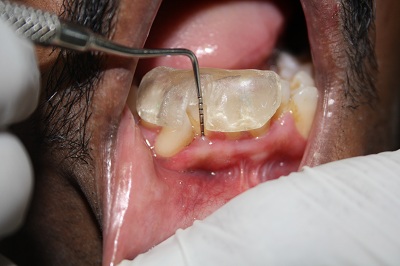
[Table/Fig-5].
Post–Operative Photograph after 120 days. Probing Pocket Depth ≤ 3mm
Discussion
Various studies have shown that conventional mechanical debridement cannot eradicate all periodontopathic bacteria from the sub–gingival environment, especially those inhabiting inaccessible areas such as furcations, grooves, concavities and deep pockets [9]. Local drug delivery systems have the ability to deliver the antimicrobial agents to the target sites, achieve sufficient concentration, and last for a sufficient duration to be effective [10]. Treatment of periodontal diseases by different types of local delivery systems has been investigated. Advantages of using the sub–gingival drug-delivery system include achieving high intra–sulcular concentrations, avoiding its systemic side effects, and better patient compliance [10]. The non-surgical therapy has covered a new dimension by addition of herbs and plant extracts to the family of local drug delivery systems.
Spirulina is a simple, one-celled, microscopic blue-green algae with the scientific name, Arthrospira platensis. Under a microscope, spirulina appears as long, thin, blue-green spiral threads. The odour and taste of spirulina are similar to those of seaweeds. It contains a rich supply of many important nutrients, including protein, complex carbohydrates, iron, and vitamins A, K, and B complexes. It also has a high supply of carotenoids such as beta carotene and yellow xanthophylls which have antioxidant properties. It is also rich in chlorophyll, fatty and nucleic acids, and lipids. Spirulina contains other nutrients such as iron, manganese, zinc, copper, selenium, and chromium. These nutrients help in fighting free radicals, cell-damaging molecules absorbed by the body through pollution, poor diet, injury, or stress. By removing free radicals, these nutrients help the immune system in fighting against cancer and cellular degeneration. In some findings, it was seen that spirulina had helped in reducing oral cancer tumours in laboratory rats, and it could thus, provide a big medical breakthrough in cancer treatment. Another health benefit of spirulina is that it stimulates beneficial flora like lactobacillus and bifidobacteria [11].
This is the first study of its kind, which was done to introduce spirulina, an algae, as non surgical therapy in chronic periodontitis subjects. Spirulina gel was used as the local drug delivery agent in the periodontal pockets of ≥5 mm, to know the anti-inflammatory effect of the gel after 120 days. This was a pioneering study which was done in this field.
Researchers have found out that the gels composed of gelatin derivatives do not appear to have the property of sustained release. Surprisingly, despite the rapid drug release and poor retention of these gels, positive clinical results were obtained in moderate to deep periodontitis [10].
Anti-inflammatory effect of spirulina on PPD and CAL
The decrease in probing depth and gain in CAL are the major clinical outcomes which are measured to determine the success of any periodontal treatment. In our study, a significant decrease in PPD was seen in both the groups, where Group A (experimental) showed a decrease from 3.36 mm to 1.79 mm and Group B (control) showed a decrease from 3.45 mm to 2.61 mm at the end of 120 days.On comparing the two groups, the decrease in PPD was found to be statistically significant, where Group A showed a greater reduction in pocket depth as compared to the Group B (control) at the end of 120 days (P≤0.05). Similarly, e gain in CAL was seen in both the groups at the end of the study, where Group A showed a gain from 4.52 mm to 3.39 mm and Group B showed a gain from 4.52 mm to 3.84mm. On comparing the two groups, Group A showed a greater gain in CAL after 3 months (p ≤0.05). The soft tissue wall changes caused by resolution of gingival inflammation explained the improvement in clinical parameters in this study. The magnitude of the changes was related to the initial pocket depth, tooth type, and other environmental factors [12].
On comparing the post–treatment values of experimental and control groups with regards to PPD and CAL, it was found that there was greater improvement in the clinical parameters in the group exposed to spirulina gel as compared to control group. In vitro antioxidant and anti-inflammatory properties of C-phycocyanin in blue green algae have been reported by various authors. The results indicated that phycocyanin was able to scavenge OH [9]. According to Romay Ch [13], González R [6], antioxidant, anti-inflammatory, neuroprotective and hepatoprotective effects have been experimentally attributed to Phycocyanin(Pc). Pc was evaluated in twelve experimental models of inflammation and it was found to exert anti-inflammatory effects in a dose-dependent fashion. Thus, according to Romay et al.,[13], Pc reduces oedema, histamine (Hi) release, myeloperoxidase (MPO) activity, levels of prostaglandin (PGE2) and laeukotriene (LTB4) in the inflamed tissues. These anti-inflammatory effects of Pc may be caused by its scavenging properties towards oxygen reactive species (ROS) and its inhibitory effects on cyclooxygenase 2 (COX-2) activity and on histamine (Hi) release from mast cells. Reddy et al., [14], by using two related assays systems (isolated enzyme assay and whole blood assay), demonstrated that Pc was a selective inhibitor of COX-2. In an isolated enzyme assay which used human recombinant COX-2, the IC50 value obtained for the inhibition of COX-2 by Phycocyanin was much lower (180 nM) as compared to those of the drugs, celecoxib (255 nM) and rofecoxib (401 nM), two well-known selective COX-2 inhibitors.
In our study, there was a significant improvement in the clinical parameters such as clinical attachment levels and probing pocket depth in the experimental group treated with spirulina gel. This was similar to the study done by Bhat et al., [15], who used aloe vera gel as local drug delivery in chronic periodontitis patients, in which probing pocket depth showed a similar improvement after the application of the gel. It is known that the periodontal disease is a chronic inflammatory infection which is associated with gram negative microorganisms that exist in sub–gingival biofilms. During periodontitis, various pro-inflammatory cytokines such as IL-1, IL-6 and TNFα are released, leading to periodontal destruction. Spirulina inhibits the activation of nuclear transcription factor which is activated by Reactive Oxygen Species (ROS), with the subsequent induction and expression of various cytokines and enzymes such as TNF and iNOS, respectively, which are involved in the induction and development of inflammatory diseases [16]. Pc exerts inhibitory effects on the release of TNF alpha, other cytokines and leukotrienes, [17] especially LTB4, which has potent chemotactic properties on neutrophils. Pc in spirulina is the main anti-inflammatory and anti-arthritic agent in microalgae, which exerts strong anti-inflammatory effects [18]. In addition, Panigrahi et al., showed a significant improvement in the wound healing activity with use of spirulina in an vitro study [19].
One of the advantages of using spirulina gel as local drug delivery, is that unlike other NSAIDs, spirulina seems to possess low toxicity and lack of adverse effects [20]. The favourable characteristics of Pc are mainly as follows:
A pronounced cyto and tissue-protective potential, in particular against oxidative stress, which is strongly involved in inflammatory, neurodegenerative and reperfusion injury disorders among others.
An anti-inflammatory potential which is caused by a set of multi-site actions such as, scavenging of various ROS, anti-lipoperoxidative and inhibitory effects on both pathways of AA metabolism (COX-2 inhibitor), inhibition of Hi release from mast cells and inhibitor of TNF-alpha), taking into account that Pc is a major constituent of microalgae Spirulina . It might exert the therapeutic effects when it is administered alone or included in the microalgae used as dietary supplement [18].
Conclusion
Scaling and Root Planning plus locally delivered spirulina gel proved the efficacy of the product as a local drug delivery system in the treatment of chronic periodontitis. Among those done on antio–xidative agents, this was the first study of its kind which used green algae as a local drug delivery for non-surgical treatment of chronic periodontitis. Spirulina appears to be promising. It exerts strong anti-inflammatory effects which are closely connected with its antioxidative activity, low toxicity and lack of adverse effects. These observations may give new direction in the field of periodontal regeneration, to achieve the goal of regeneration without any invasive procedures, thereby causing less discomfort to the patients. However, since this is the first study of its kind in this field, further cross-sectional and longitudinal studies using different vehicles and concentrations of Spirulina, are needed to further affirm the association of spirulina gel with periodontal health.