Myofibroblastoma (MFB) is a rare mesenchymal tumour, derived from mammary stromal fibro-myofibroblasts, with diverse biological and morphological behaviour. Large and cellular myofibroblastomas, especially those with epitheliod like cells, can mimic various spindle cell lesions and metaplastic carcinomas, thus posing diagnostic challenge. A 50–year woman presented with slow growing, painless lump in the left breast. Fine Needle Aspiration (FNA) smears showed predominant atypical spindle cell population, pleomorphic epithelial like cells and giant cells. Cytodiagnosis of atypical spindle cell lesion with the possibility of metaplastic carcinoma was suggested. Histopathological examination showed fascicles of spindle cell population admixed with epithelial like cells, atypical cells and tumour giant cells, thus raising differential diagnosis of metaplastic carcinoma, low grade spindle cell sarcoma and myofibroblastic tumour. Lymph nodes were negative for metastatic deposits. Immunohistochemistry revealed variable coexpression of markers for vimentin, fibronectin, CD34, SMA (smooth muscle actin), but negative expression for , S-100, CD99, CK7 (cytokeratin 7), HMWK (high molecular weight keratin), ER (oestrogen receptor) and PR(progesterone receptors). Diagnosis of cellular myofibroblastoma with mixed unusual morphological features was defined, based on both histological and immunohistochemical features. MFB may cause a potential diagnostic pitfall while interpreting FNA and histopathological sections due to its wide differential diagnosis. The distinction of MFB from its cytohistological mimics of malignancy is crucial to avoid unnecessary extensive procedures. The case report emphasizes the role of immunohistochemistry as gold standard in diagnosis of MFB. The case is also being presented because of its large size and rare mixed unusual morphological features.
Introduction
MFB is prototypical of stromal tumours of the breast, comprising neoplastic cells showing a variable fibro-myofibroblastic differentiation at morphological, immunohistochemical, and ultrastructural levels. The tumour, Myofibroblastoma, was originally reported in 1981 by Toker et al., as spindle cell lesion of the male breast; associated with gynaecomastia. Subsequently, cases have also been reported in female breast, indicating its occurrence in both sexes [1] The term, myofibroblastoma was coined by Wargotz et al., in 1987 who labelled it as distinct clinicopathological entity. Till 2007,70 cases have been reported in literature [2,3] but more cases are being diagnosed due to advanced diagnostic techniques including mammography.
Besides breast, lesion has been described in soft tissues, skin, tongue, lymph nodes and suprasellar region [4,5]. It is usually seen in the sixth to seventh decade of life, with sizes varying from few millimetres to 11cm, average being 5cm [6]. Due to variable fibro-myofibroblastic differentiation, many histomorphological forms are seen. Their recognition as distinct entities is crucial, for excluding both histologically mimicking benign or malignant breast neoplasms.
Case Report
Clinical History
A 50-year-old female presented with a lump in left breast, with eight months growth. On examination well-defined, firm, non-tender lump measuring 15x11 cms, non adherent to overlying skin was identified. Lymph nodes were non palpable.
Cytomorphological Examination
The lump was aspirated and the alcohal fixed smears were stained with H and E and Giemsa stains. Hypercellular smears were composed mainly of bland spindle to oval cells with scanty cytoplasm, arranged randomly in clusters and dispersed singly.[Table/Fig-1] The cells showed mild degree of pleomorphism and hyperchromatism. Few epithelial like round to oval pleomorphic cells with hyperchromatic nuclei, having abundant eosinophilic cytoplasm, were seen [Table/Fig-2] with multinucleated and occasional bizzare cells. Cytodiagnosis of atypical spindle cell lesion or metaplastic carcinoma was given. Patient underwent radical mastectomy and the specimen was sent for histopathological examination.
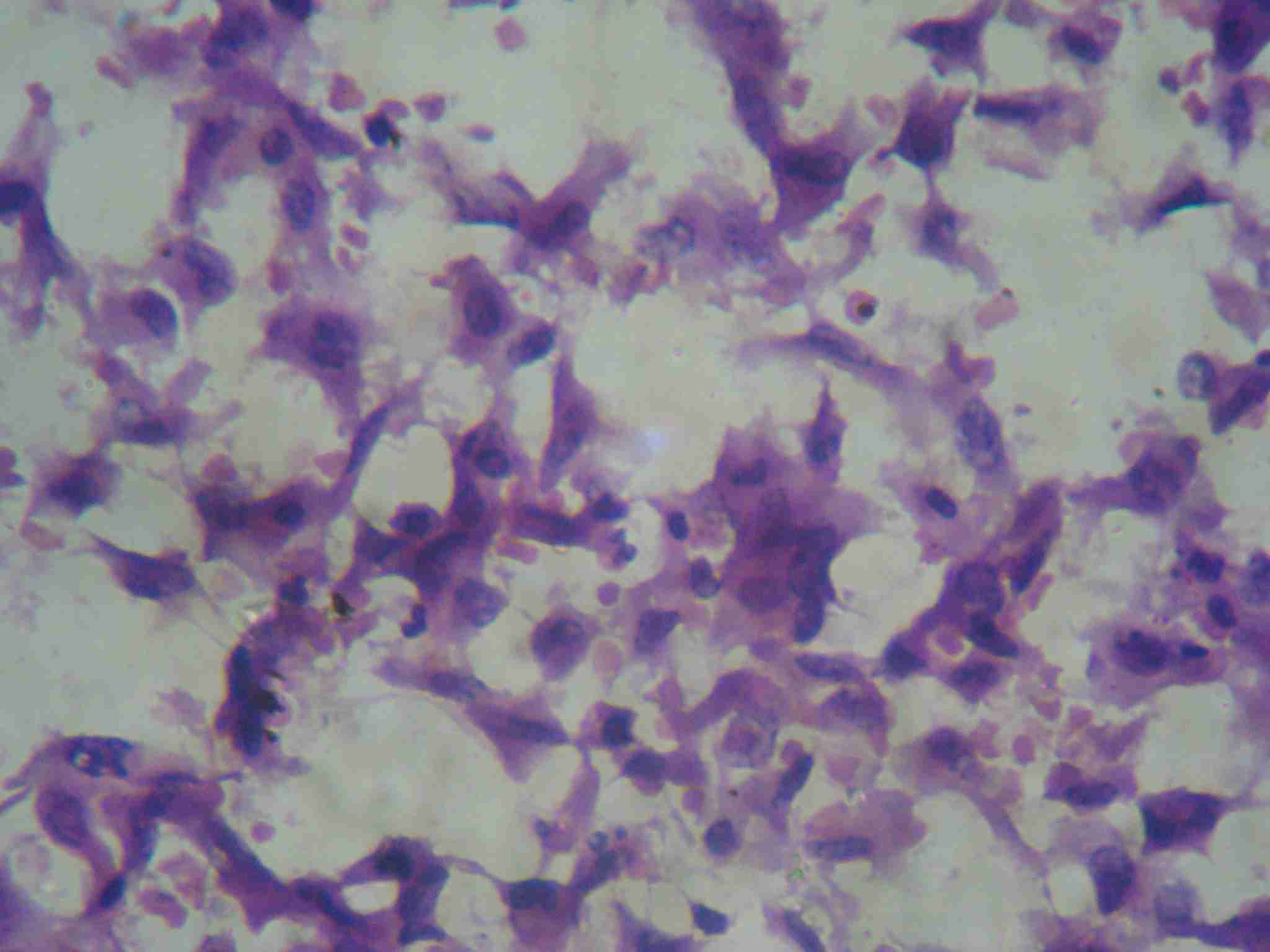
Cytological smear showing spindle cell population with oval nuclei showing mild pleomorphism (H&E staining 40X)
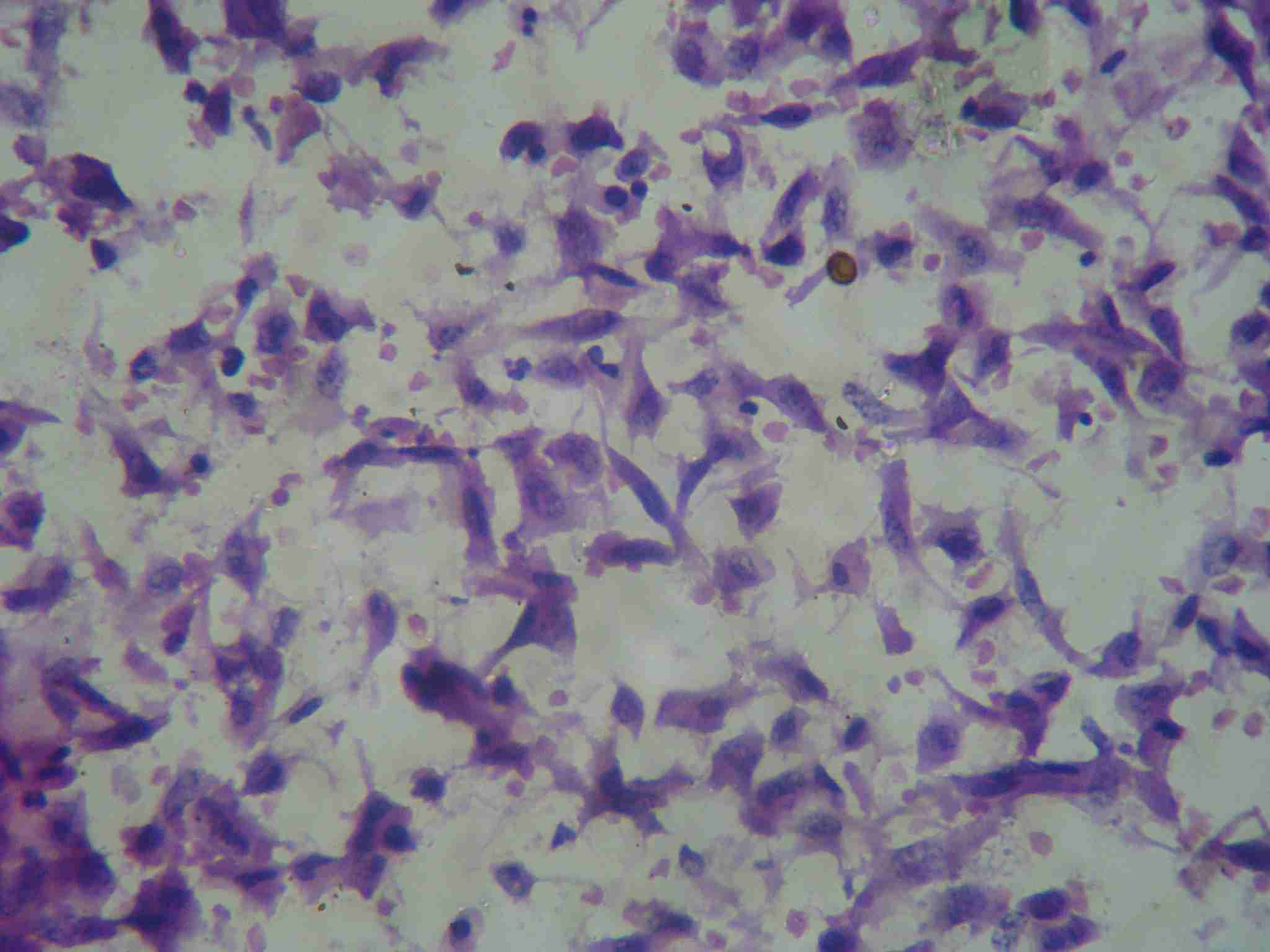
Cytological smear showing predominant spindle cell population with few epithelial like cells having abundant cytopalsm and eccentric nuclei (H&E staining 40X)
Gross Examination:- Specimen of breast measuring 15x11x8 cm, with growth measuring 13x11x9 cm, was identified. Overlying skin was non adherent, without nipple retraction. Two lymph nodes were recovered from axillary tail.
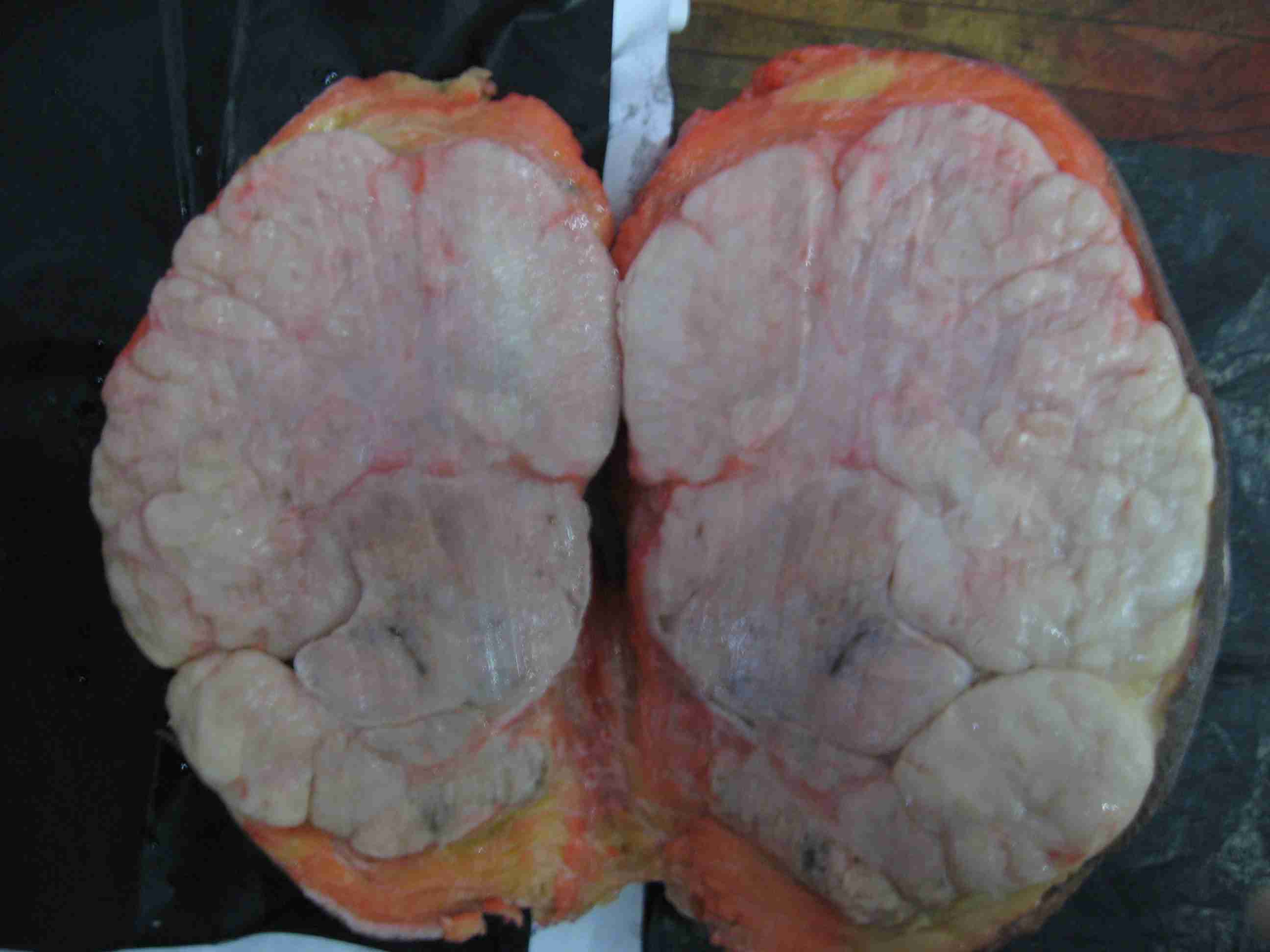
Cut section:- Uncapsulated and lobulated grey white area of growth with pushing margins, measuring 13 x 11 x 8cm, compressing adjacent breast tissue, was identified [Table/Fig-3]. Necrosis and cystic changes were absent.
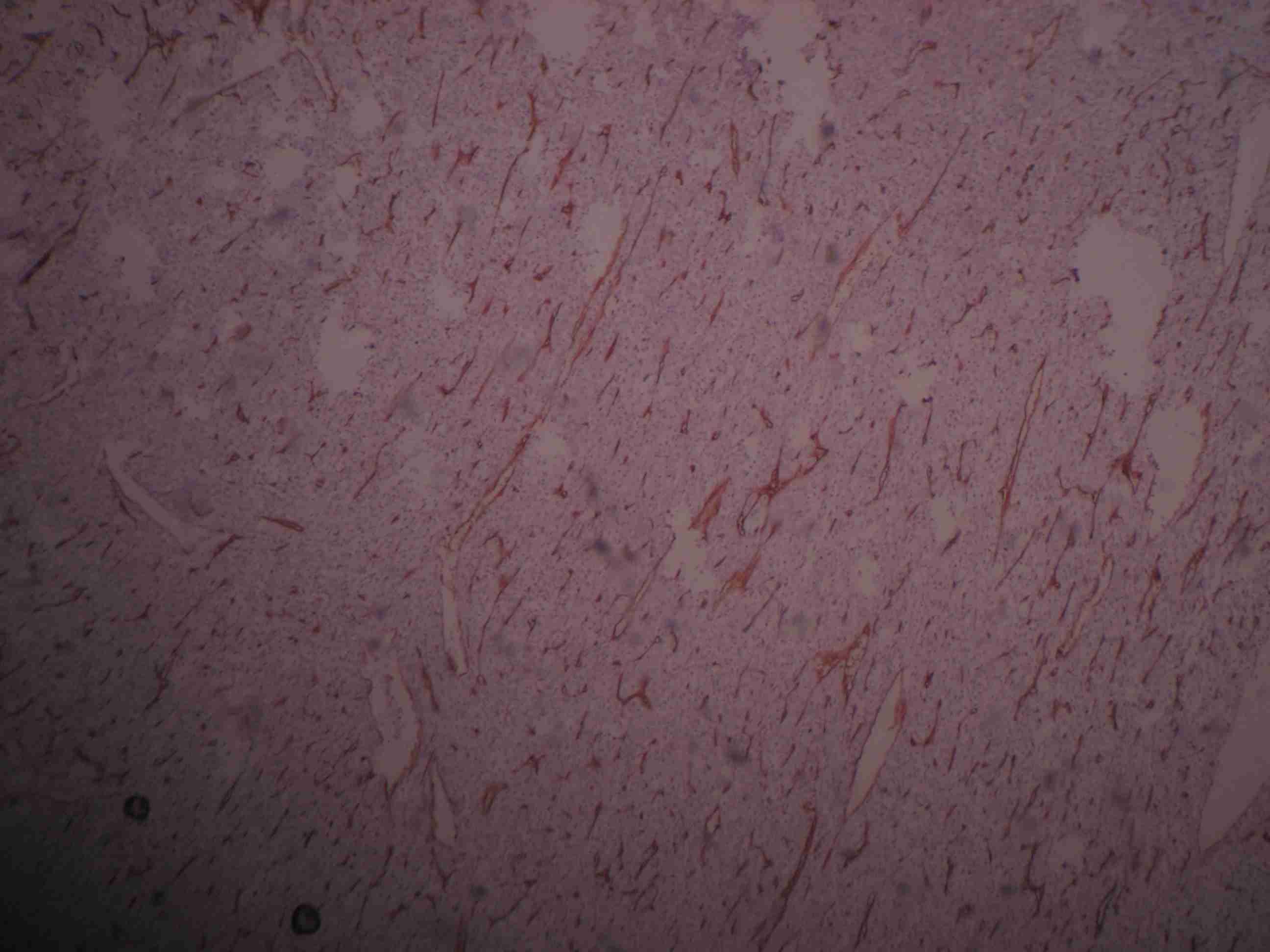
Cut section of the speciemn of breast shows uncapsulated and lobulated grey white area of growth with pushing margins, measuring 13 x 11 x 8, compressing adjacent breast tissue (H&E staining 40X)
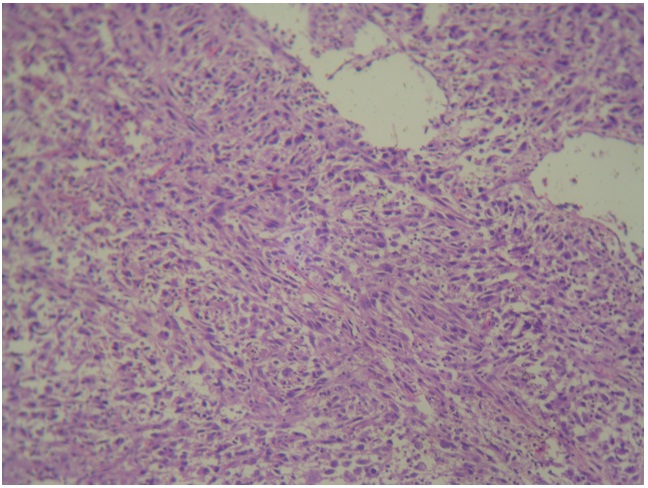
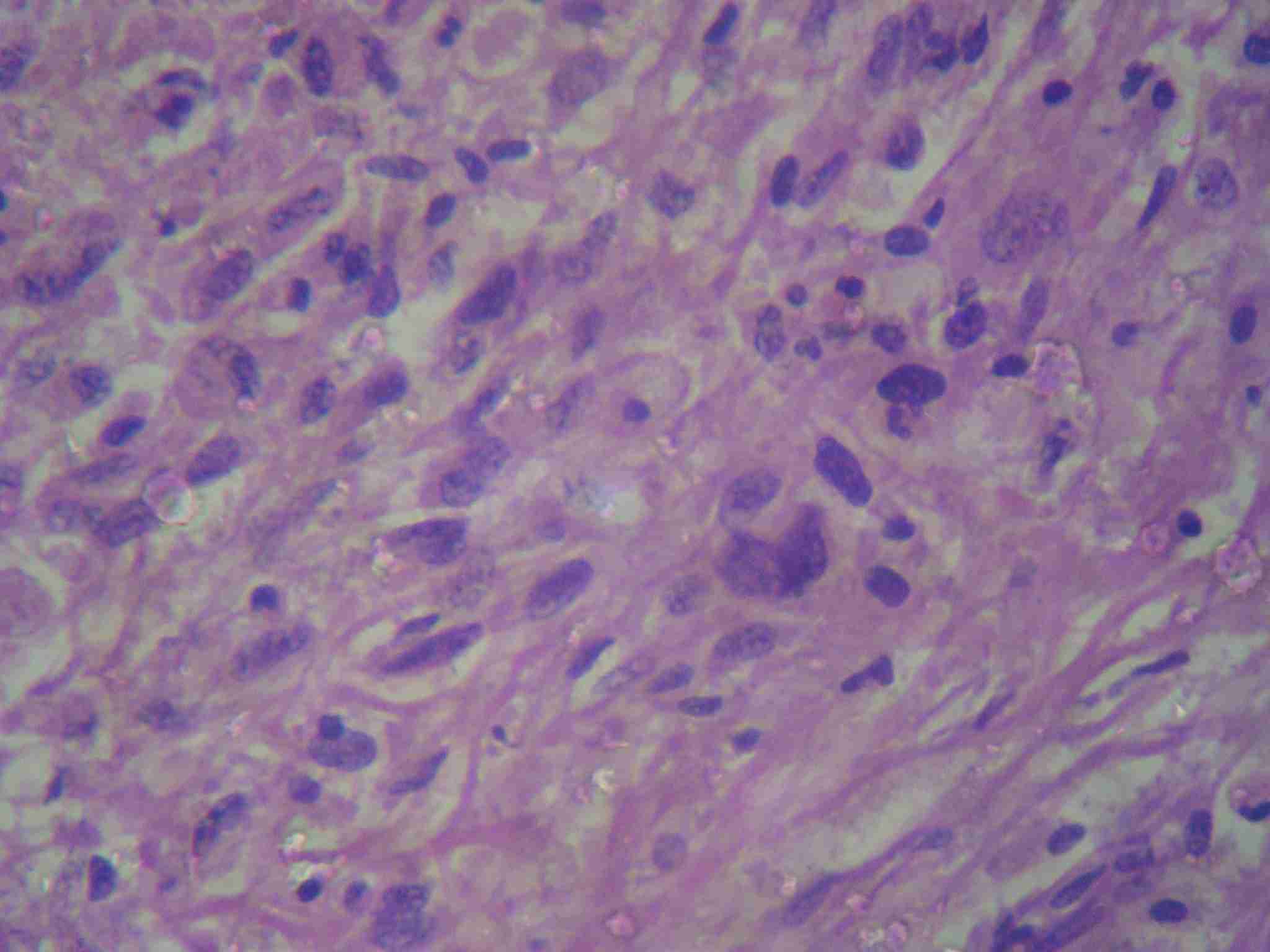
Microscopically:- H and E stained sections from the growth revealed predominant population of bland to pleomorphic spindly cells arranged in fascicles, showing variable degree of hyperchromatism [Table/Fig-4]. Focally, collagen laying was seen with mitotic figures <2/ 10HPF. At places, cells arranged in hemangiopericytoma like pattern were also seen. Many round to oval cells with abundant eosinophilic cytoplasm, pleomorphic hyperchromatic nuclei are seen amidst spindly cell population and constituting <20% of tumour mass [Table/Fig-5,6]. Multinucleated cells and giant cells were also present along with focal lymphoplasmacytic infiltrate. Normal breast ducts were seen entrapped in tumour tissue. Lymph nodes showed reactive pathology. Histological appearances were compatible with a wide differential diagnosis including, metaplastic carcinoma, myoepithelial–epithelial lesions, myofibroblastoma, dermatofibrosarcoma protuberance with peripheral nerve sheath tumour. Histological diagnosis of atypical spindle cell lesion, especially myofibroblastoma or metaplastic carcinoma, was given. Immunohistochemical evaluation was suggested.
Histological sections showing mixed tumour cell poplation with spindle cells arranged in fascicles, hemangipericytoma like pattern separated by groups of epithelial and inflammatory cells. (H&E staining 10X)
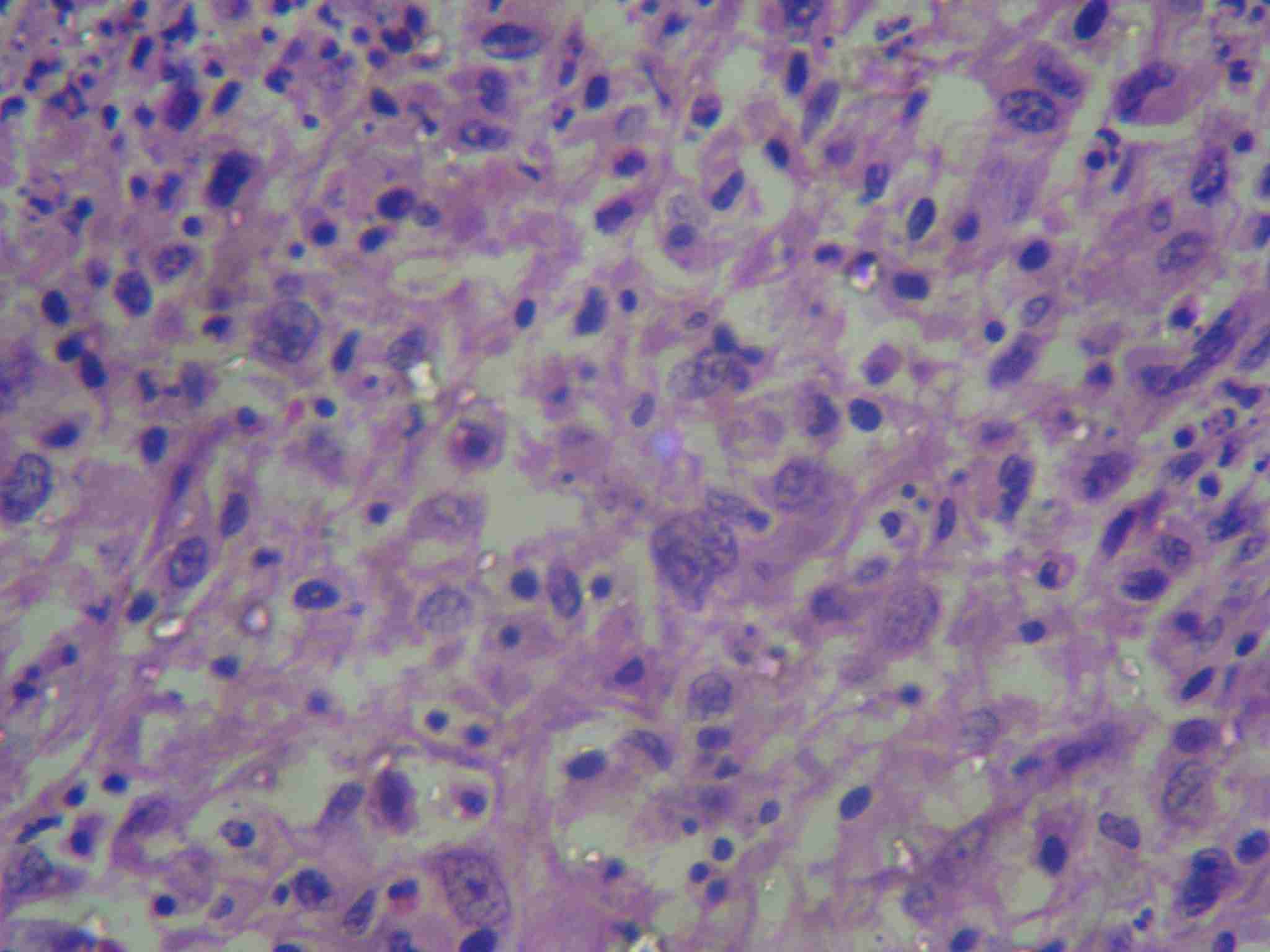
Histological sections showing predominant spindle cell population with few epithelial like cells having abundant cytopalsm and eccentric nuclei (H&E staining 40X)
Immunohistochemical Findings:- On immunohistochemical examination, sections were found to be highly positive for vimentin and CD99, focally positive for smooth muscle actin (SMA) and CD34, [Table/Fig-7, 8], while complete negativity was seen for S-100, cytokeratin 7 (CK7), high molecular weight keratins (HMWK), oestrogens receptors (ER) and progesterone receptors (PR). Diagnosis of myofibroblastoma was given after correlating histopathological and immuno-histochemical findings.
Histological sections showing epithelial like cells having abundant cytopalsm and eccentric nuclei showing variable pleomorphism and hyperchromatism amidst spindle cell population and inflammatory infiltrate(H&E staining 40X)
Histological sections showing tumour cells diffusely positive for vimentin (IHC stain 40x)
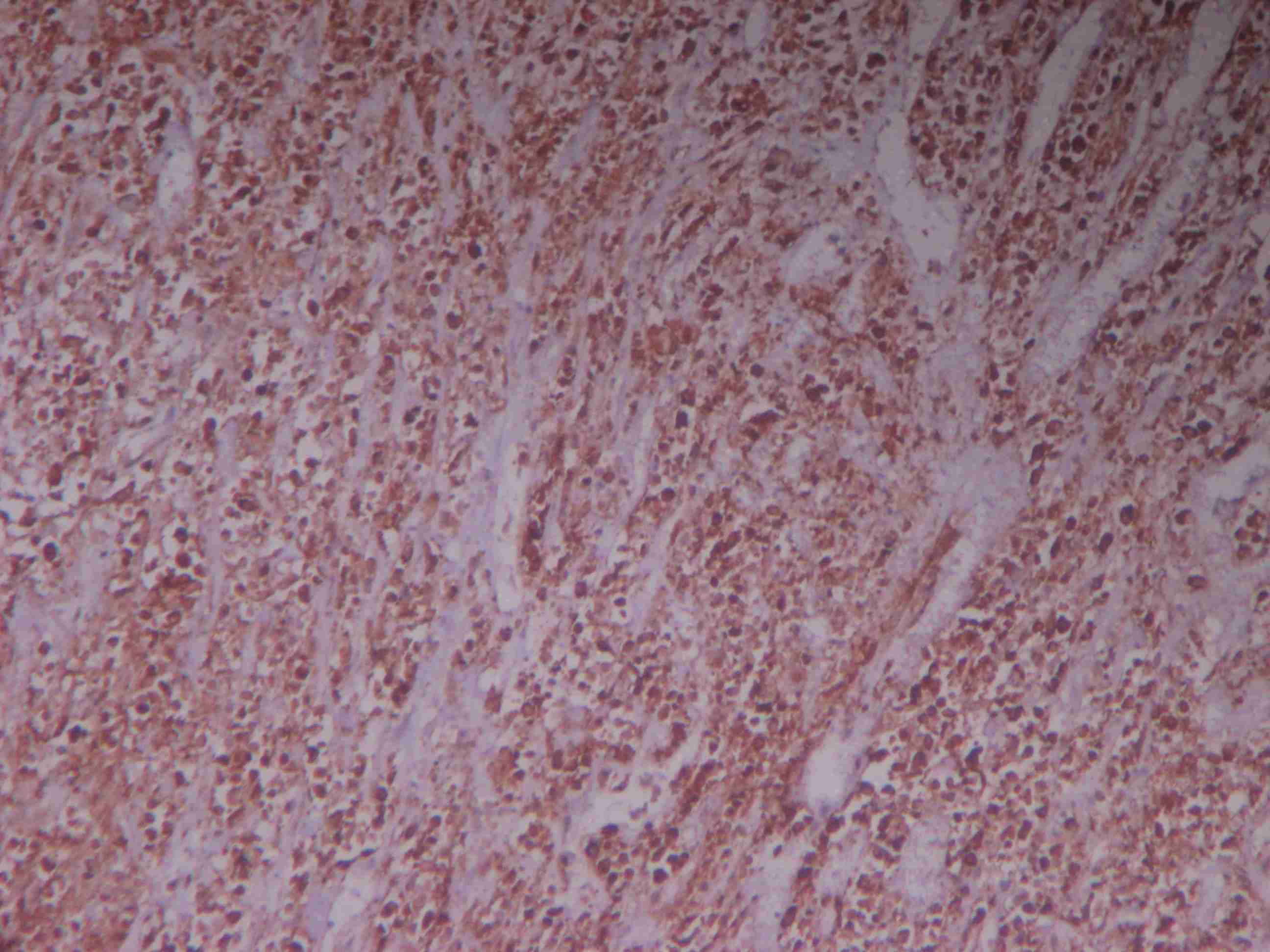
Histological sections showing tumour cells showing variable positivity for CD34 (IHC stain 40x)
Discussion
Myofibroblastomas are rare soft tissue tumours. As neoplastic cells show myofibroblastic differentiation, these are thought to be derived from stromal fibroblasts and mammary myoepithelial cells. They usually present as solitary lesions. Cases reported in the literature were mostly seen in men of ages ranging from 41-85 years, unilateral, unencapsualted lesions with variable histological patterns, interlesional and intralesional morphology [7]. Current case was reported in a 50-year-old female as slow growing mobile lump not associated with pain. Cytologically, MFB in aspirated material predominantly appears as cellular smear with monomorphic bland to slightly atypical spindly cell population. Cells adherent to vascular strands may give features of wide range of spindle lesions with marked angiogenesis or reminiscent of vascular lesions [8]. Current case also revealed highly cellular smears with monomorphic population of spindled cells. At places, scattered oval to round epithelial like cells with pleomorphic, hyperchromatic nuclei, with moderate amount of eosinophilic cytoplasm can be seen amidst predominant spindle cell population. However, exact nature of spindle cells, whether of epithelial or mesenchymal origin, cannot be ascertained from cytology alone; so, exact diagnosis was difficult to be made on cytology. Keeping in view the above features, possibilities of metaplastic carcinoma and atypical spindle cell lesions were considered. Classically, on histological sections, MFB is composed of spindle cells in fascicles, which exhibit varying degrees of myogenic and fibroblastic differentiations and is invariably traversed by collagen fibres. Infiltrative margins with entrapped benign breast glands can be identified in minority of the cases. Mitoses is either absent or 2-3/ HPF. Small-to-medium sized vessels along with mast cells, focal lymphoplasmacytic infiltration can be occasionally detected [1,7].
In the present case report, H and E stained sections showed predominant bland to slightly pleomorphic spindle cell populations arranged in groups and fascicles, with infiltrative pattern at places. At places, hemangiopericytoma like pattern was also seen, with increased and congested blood vessels. Small amount of entrapped normal breast tissue was seen at the tumour periphery. Under high power, tumour cells mainly spindly showed mild degree of pleomorphism and hyperchromatism, with scanty cell cytoplasm. Spindle cells showed variable degrees of collgenization, thus imparting alternating hypercellular and hypocellular appearances. Seen amidst spindle cell population, were groups of pleomorphic round to oval cells with hyperchromatic nuclei and moderate amount of eosinophilic cytoplasm. Multinucleated cells with tumour giant cells, along with small focal areas of necrosis with lymphoplasmacytic infiltrates, were seen. Mitotic figures were < 2/HPF. From the above histological picture, possibilities of fibrous tumour, metaplastic carcinoma, nerve sheath tumour with epithelioid cells were thought of. The case was subjected to immunohistochemical examination for the final diagnosis.
Despite their presentations as different cell types, growth patterns and variations in extracellular matrix composition, MFBs retain their basic characteristics, which helps in their recognition, thus avoiding diagnostic confusion which arises from various benign and malignant histological mimics. Hence, immunohistochemistry plays a crucial role in their definite diagnosis and helps in differentiating MFBs from other spindle cell lesions e.g myoepithelial lesions, solitary fibrous lesions, nodular fasciitis, fibromatosis breast, nerve sheath tumours, hemangiopericytoma, malignant phyllodes. As per literature, immunohistochemically, most cases of MFBs are positive for vimentin, CD34, α-smooth muscle actin, bcl-2, and CD99, but with variable expressions in different tumours and also in different areas of the same tumour. CD68 and factor XIIIa immunoreactivity and CD10 have been documented in some cases, depicting that their origin was myoepithelial cells. Cytokeratins, EMA, S100 protein, HMB-45, and c-Kit (CD117) are consistently negative [1,9,10].
In the present case, neoplastic tumour cells were highly positive for vimentin, CD99, focally positive for SMA, CD34 and negative for CK7, HMWK, ER, PR, S-100. Negative staining with cytokeratins and immunoreactivity with vimentin and CD99, variably with smooth muscle actin and CD34, help in excluding metaplastic carcinomas. S100 negativity helps in excluding nerve sheath tumours. Presence of epitheioid like cells excluded phyllodes. As present lesion showed focal positive immunoreactivity for SMA but negativity for cytokeratins , it can be proposed that this lesion could be showing differentiation towards myoid cell lines. Hence, positivity for SMA, ME cell nature can be excluded, due to negative HMWK staining. Possibilities of extrapleural solitary fibrous lesion and myofibroblastic lesions, including MFB and low grade myofibroblatic sarcoma, were thought of. Though these lesions are indistinguishable from solitary fibrous tumours, some authors believe that immunohistochemically and histopathologically, the two lesions as one and same thing, which was in concordance with findings of other studies [11]. But solitary fibrous tumours were well encapsulated in this case, no discernible capsule was seen, thus favouring the diagnosis of myofibroblastic lesion.
Large number of histomorphological variants have been identified, including both common and unusual variants. Common variants include a) Cellular MFB showing storiform / herring bone patterns with infiltrative margins. b) Epithelioid MFB in which epithelioid cells constitute >50% of neopalstic cells. Medium-sized mononucleated, binucleated, or multinucleated neoplastic cells with well-defined cell borders, oval to polygonal in shape, with abundant eosinophilic cytoplasm and round to oval eccentrically placed nuclei containing small evident nucleoli, are usually arranged in clusters or in alveolar, solid, or trabecular growth patterns. They are variably embedded in a myxoid-to-fibrous stroma and show evidence of collagenisation.(c) Deciduoid MFB (d) Infiltrative MFB (e) Lipomatous MFB (adipose tissue constituting >75% of tumour cells) (f) Myxoid MFB (g) Collagenized MFB (h) Mixed variant. Unusual variants include MFB with atypical cells, with multinucleated floret like cells, with hemangiopericytoma like pattern and with heterlogous mesenchymal component, including cartilage, osseous components, epithelial like cells , giant cells and infiltrative margins. In the present case, diagnosis of cellular myofibroblastic lesion with mixed histomorphological features was made.. Our case could not be designated as epithelioid MFB, as in current case, epithelioid like cells constituted only 10-20% of tumour population. Features of mild pleomorphism, hyperchromatism and necrosis point towards atypical myofibroblastic lesions and low grade myofibrosarcomas. So far ,in the very few reported cases of myofibrosarcoma, intracytoplasmic hyaline inclusion bodies with mitotic figures > 3/HPF are noted [12]. In this reported case, no intracytoplasmic hyaline bodies or fibroid bodies with occasional mitotic figures were seen. Small areas of necrosis can be attributed to history of fine needle aspiration from the same lesion and hence, the inflammatory infiltrate. In the current case, immunoprofile, along with histological features, favour the diagnosis of myofibroblastoma. Thus, immunohistochemistry is extremely helpful in confirming the diagnosis of MFB. The patient was followed for one year after surgery, but no recurrence was noted, suggesting indolent nature of the lesion.
Conclusion
MFB is a rare mesenchymal lesion of the breast, with wide variations in biological behaviour, usually running benign course and very rarely prone to recurrence and metastases, which has to be differentiated from its histological mimics. Immunohistochemistry plays a major role in making the correct diagnosis.
[1]. Gaetano M, Mammary myofibroblastoma: A Tumor With a Wide Morphologic SpectrumArch Pathol Lab Med 2008 132:1814-20. [Google Scholar]
[2]. Wargotz ES, Weiss SW, Norris HJ, Myofibroblastoma of the breast: sixteen cases of a distinctive benign mesenchymal tumorAm J Surg Pathol 1987 11:493-502. [Google Scholar]
[3]. Sharma A, Sen AK, Chaturvedi NK, Yadav R, Myofibroblastoma of male breast: a case reportIndian J Pathol Microbiol 2007 50:326-28. [Google Scholar]
[4]. Sahin AA, Ro JY, Ordonez NG, Luna MA, el-Naggar AK, Goepfert H, Myofibroblastoma of the tongue. An immunohistochemical, ultrastructural, and flow cytometric studyJ Clin Pathol 1990 94(6):773-77. [Google Scholar]
[5]. Shinojima N, Ohta K, Yano S, Nakamura H, Kochi M, Ishimaru Y, Myofibroblastoma in the suprasellar region. Case reportJ Neurosurg 2002 97(5):1203-07. [Google Scholar]
[6]. Qureshi A, Kayani N, Myofibroblastoma of breast 2008 51(3):395-96. [Google Scholar]
[7]. Reis-Filho JS, Faoro LN, Gasparetto EL, Totsugui JT, Schmitt FC, Mammary epithelioid myofibroblastoma arising in bilateral gynecomastia: case report with immunohistochemical profileInt J Surg Pathol 2001 9:331-34. [Google Scholar]
[8]. Schmitt FC, Mera AC, Fine Needle Aspiration Cytology Presentation of a Cellular Variant of Breast Myofibroblastoma: Report of a Case with Immunohistochemical StudiesActa Cytol 1998 42:721-24. [Google Scholar]
[9]. Das P, Sharma A, Arora R, Singh MK, Myofibroblastoma of BreastIndian J Med and Paed Oncol 2008 29(3):16-19. [Google Scholar]
[10]. Santander RL, Nieto GS, Gozalvo AC, Holguin VC, Roman VJ, Myofibroblastoma of BreastVirchows Arch 1999 434:547-50. [Google Scholar]
[11]. Salomão DR, Crotty TB, Nascimento AG, Myofibroblastoma and solitary fibrous tumour of the breast: histopathologic and immunohistochemical studiesBreast 2001 Feb 10(1):49-54. [Google Scholar]
[12]. Morgan PB, Chundru S, Hatch SS, Hawkins HK, Adegboyega PA, Eltorky MA, Low-Grade Myofibroblastic Sarcoma of the BreastJournal of Clinical Oncology 2005 23(25):6249-45. [Google Scholar]