Paraplegic Neurodeficit Management Post Endovascular Graft: A Rare Case of Aortic Dissection
Vilas Yadavarao Kanse1, Dhanaraj Singh Chongtham2, S C Nemichandra3, Kenny Singh Salam4
1 Post Graduate Student, Department of Medicine, Regional Institute of Medical Sciences, Imphal, Manipur, India.
2 Professor, Department of Medicine, Regional Institute of Medical Sciences, Imphal, Manipur, India.
3 Post Graduate Student, Department of Medicine, Regional Institute of Medical Sciences, Imphal, Manipur, India.
4 Senior Resident, Department of Medicine, Regional Institute of Medical Sciences, Imphal, Manipur, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kanse Vilas Yadavarao, # 4 Postgraduate Gents hostel 5A, Regional Institute of Medical Sciences, Imphal-795004, Manipur, India.
Phone: 09862830616,
E-mail: vilas_medi@yahoo.co.in
Acute aortic dissection is a catastrophic episode that usually presents as a sudden, painful, ripping sensation in the chest or back. It is associated with neurologic sequelae in as many as one-third of patients. We report a case of aortic dissection, presenting as acute paraplegia. A 50-year-old patient presented to us with chief complaints of paraplegia and back pain. On examination, strength was 5/5 in both upper extremities and 0/5 in both lower extremities. Deep tendon reflexes were absent in her legs. CT angiogram of aorta Aortic Dissection Stanford type B / De-Bakey type –III. Patient was treated with endovascular graft for aortic dissection, paraplegia recovered completely.
Aortic dissection, Paraplegia, Endovascular grafting, Recovery
Introduction
Acute aortic dissection is the most common catastrophic event affecting the aorta, with an estimated annual incidence of approximately 5 to 30 per million [1].
Neurologic manifestations occur in 17%-40% of aortic dissections cases, especially type A dissections. Neurologic syndromes include transient ischemic stroke, spinal cord ischemia, ischemic neuropathy, and hypoxic encephalopathy [1]. Stroke can be the presentation of aortic dissection in 10% of patients. Around 2%-5% of cases may present as paraplegia or paraperesis. Paraplegia is due to spinal cord ischemia due to separation or occlusion of intercoastal arteries from aortic lumen by the dissection flap [2].
Case Report
A 50-year-old female experienced sudden onset of severe backache, tearing type but, not radiating to lower limbs. After 20 minutes patient had noticed weakness in her both lower limbs, to start with on left lower limb which progressed to right lower limb in 10 minutes. Weakness was sudden in onset involving both proximal and distal group of muscles. No association was found with upper limbs, facial weakness or bowel and bladder involvement. No loss of consciousness, trauma or fall. On examination, in emergency medical department, BP was 230/130mmhg, pulse 102/min regular, pulse volume was decreased in right lower limb, conscious & well-oriented, no cranial nerve involvement. Motor system power 0/5 both lower limbs, reflexes absent, planter reflex was mute and sensations diminished. Other systems were normal. Weakness in left lower limb recovered in next 24 hours, right lower limb weakness persisted.
Cervicodorsolumbar spine MRI was normal. Echo suggested dilated root of aorta, ascending and descending aorta. Patient underwent CT angiogram of aorta which was suggestive of Aortic Dissection, Stanford type B / De-Bakey type –III [Table/Fig-1].
CT angiogram of aorta which was suggestive of Aortic Dissection, Stanford type B / De-Bakey type –III
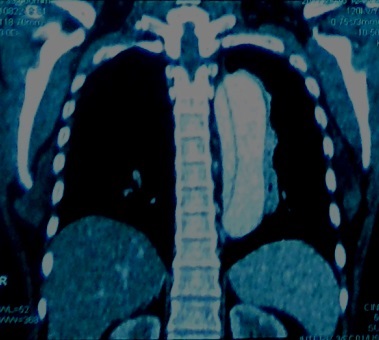
Neurology consultation was taken and predominantly right-sided ischaemic lumbosacral plexopathy was diagnosed. The patient was referred to a higher centre at CMC, Vellore where she underwent endovascular repair of Type B aortic dissection with chimney graft preservation of the left subclavian artery under general anesthesia. Post-surgery weakness in right lower limb improved dramatically. Finally the patient was able to walk without support at the time of discharge.
Discussion
The spinal cord receives blood from three longitudinal channels, one anterior spinal artery (ASA) and two posterior spinal arteries (PSA). Feeding arteries from the thoracic aorta provide interrupted segmental supply to the ASA and PSAs. Normally two small radicular branches supply the cord between C8 and T9; the remainder of the spinal cord is supplied largely by the arteria radicularis magna (artery of Adamkiewicz) with minor contribution from an infrarenal radicularartery. The artery of Adamkiewicz, originating from the left in 80% of the population, arises from T5-T8 in 15% of cases, T9-T12 in 75%, and L1-L2 in 10% [2,3].
Acute aortic dissection is the most frequently fatal condition in the spectrum of chest pain syndrome. Incidence of aortic dissection is 2.9/100,000/year [1,4]. Dissection may compress or occlude a branch of the aorta and produce acute ischemia. Ischemia occurs in arms or legs in 20% of patients, in the kidney in 15%, myocardium in 10%, brain in 5%, and mesentery or spinal cord in 3% [4,5]. Risk factors predisposing for aortic dissection were Systemic hypertension in 52% with type I or II dissection and in 75% with type III dissection. 44% and 18% of patients with the Marfan syndrome and Cystic medial necrosis suffered from aortic dissection respectively [6].
In the present case, patient was known hypertensive on irregular treatment. She presented with sudden onset of paraplegia following severe backache. The patient was having high BP unequal pulse volume and LMN type of flaccid paraplegia. With these findings the case was suspected to be of aortic dissection presenting as paraplegia. Patient underwent CT angiogram of aorta suggestive of Aortic Dissection, Stanford type B / De-Bakey type –III. We think distention of the false lumen with blood causes the intimal flap to compress on the true lumen of arteria radicularis magna (artery of Adamkiewicz), narrowing its caliber and distorting its shape, or seperating it from true lumen which leads to malperfusion dorsal spine and resulted in paraplegia.
With initial measure to control BP with antihypertensives, weakness improved in both lower limbs predominatly in left lower limb.The patient was planned for endovascular graft repair Type B aortic dissection with chimney graft preservation of the left subclavian artery under general anaesthesia. The post-surgery weakness in right-lower limb improved dramatically.
Though paraplegia presenting as a complication of aortic dissection is rare (2%-5%) but many cases have been reported, most of these patients have been treated conservatively and paraplegia was permanent.
Necmettin Colak et al., treated a patient who had paraplegia due to aortic dissection, with lumbar catheter which was inserted for cerebrospinal fluid drainage, and axillary arterial cannulation was established. With the use of cardiopulmonary bypass, the aortic dissection was corrected. The surgery restored spinal and lower-extremity perfusion, and the patient walked unaided from the hospital upon his discharge 5 days later [7]. James I Fann et al., operated on 9 patients of which 4 patients died of myocardial infaraction and respiratory tract infection, remaining 5 patients had gradual recovery of spinal cord injury [8].
Conclusion
The present case reported is of aortic dissection presented along with sudden-onset paraplegia. The patient fully recovered after emergency surgery and had no neurologic deficits. Although paraplegia in association with acute aortic dissection is rare, aortic dissection should be considered in the differential diagnosis of patients who present with acute-onset flaccid paraplegia (whether painful or painless) with decreased volume of femoral arteries bilaterally. These cases should be treated aggressively considering patient’s critical condition & related requirement.
[1]. Bonow RO, Mann DL, Zipes DP, Libby P, Braunwald’s Heart Disease A Textbook of Cardiovascular Medicine 2012 9thedElsevier saunders Publisher:1319-31. [Google Scholar]
[2]. Kirklin JW, Boyes BGB, Cardic surgery 1993 2nd edChurchill Livingstone Publisher [Google Scholar]
[3]. Shamji MF, Maziak DE, Shamji FM, Ginsberg RJ, Pon R, Circulation of the Spinal Cord: An Important Consideration for Thoracic SurgeonsAnn Thorac Surg 2003 76:315-21. [Google Scholar]
[4]. István Mészá ros, Jó zsef Mó rocz, Jó zsef Szlá vi, János Schmidt, Lá szló Tornó ci, Lá szló Nagy, Epidemiology and Clinicopathology of Aortic Dissection* A Population-Based Longitudinal Study Over 27 YearsCHEST 2000 117:1271-78. [Google Scholar]
[5]. Prêtre R, Segesser LKV, Aortic dissectionLancet 1997 349:1461-64. [Google Scholar]
[6]. Larson EW, Edwards WD, Risk factors for aortic dissection: A necropsy study of 161 casesThe American Journal of Cardiology 1984 Vol 53(Issue 6):849-55. [Google Scholar]
[7]. Colak N, Nazli Y, Alpay MF, Akkaya IO, Cakir O, Painless Aortic Dissection Presenting as ParaplegiaTex Heart Inst J 2012 39(2):273-6. [Google Scholar]
[8]. Fann JI, Sarris GE, Mitchell RS, Shumway NE, Stinson EB, Oyer PE, Treatment of Patients with Aortic Dissection Presenting with Peripheral Vascular ComplicationsAnn Surg 1990 212(6) [Google Scholar]