Diabetic foot ulcer is common and it is estimated to affect 15% of all diabetic individuals during their lifetime. Prevalence of diabetic foot ulcer in clinical population is 3.61%. Diabetic foot ulcers precede almost 85% of amputations in India [1]. Management of the diabetic foot requires a multisystem approach, which includes the nervous, vascular, skeletal, immune, and integumentary systems [2].
The pathophysiological changes in multiple organ systems are the result of metabolic dysregulation associated with diabetes mellitus, imposes tremendous burden on individual [3]. For many decades, various different techniques have been tried to treat chronic leg ulcers, but none was proved to be ideal dressing. Phenytoin has been used by many workers because of its positive effects in ulcer healing, such as increase in the proliferation of fibroblasts and deposition of collagen, neovascularization, enhanced granulation tissue formation, decrease in the action of collagenase and bacterial contamination [4–8]. The antibacterial activity of phenytoin contributed to removal of Staphylococcus aureus, Escherichia coli, Klebsiella species, Pseudomonas [1,9–11]. Since some authors have reported use of phenytoin in healing of different ulcers including diabetic foot ulcer, it prompted us to conduct a study on the local use of phenytoin on diabetic foot ulcer healing.
Material and Methods
Patients admitted as in-patients in Department of Surgery in B.L.D.E.University’s Shri B.M. Patil Medical College Hospital, India for Grade I and II diabetic foot ulcers from October 2009 to May 2011,were included in the study. Informed consents were taken from all the participants. Institutional ethical committee approved the study design. Based on Wagner’s wound classification [12], one hundred patients with grade I/II diabetic foot ulcers were randomly divided into two equal groups (Lottery method). Details of cases were recorded, including history and clinical examination with baseline characteristics such as sex, age, average duration of diabetes, pedal pulses, peripheral neuropathic changes, neuro-ischaemic changes, etc. Routine pre-operative investigations were done in both the groups. Necessary debridement was done in all the cases. Culture and sensitivity swabs were taken from all the cases and sent for microbiological evaluation before start of the study. The required patients were treated with appropriate systemic antibiotics as per culture and sensitivity reports. Usual anti–diabetic treatment was continued in all patients. After slough removal, the surface area was measured, tracing the outline on butter paper. This outline was transferred to graph paper. On each occasion, ulcer areas were measured twice. All the measurements were carried on by a single examiner for all the participants. When they were identical, the readings were recorded. If they were not, the average was recorded. Gentle saline cleaning and topical phenytoin or control application were done and a sterile dry dressing cover was applied daily for a period of 6 weeks or until complete healing (defined as intact skin indicated as complete cure), whichever occurred earlier. Patients from both the study and control groups were compared for final analyses. Phenytoin dose estimation was done, based on the surface area. Dosage used was for, 0-5 cm2 →100 mg, 5.1-9 cm2 → 150 mg, 9.1-15 cm2 → 200 mg, >15 cm2 → 300 mg respectively. Phenytoin powder was mixed with normal saline and applied with gauze, as was described by Rhodes [13].
Patients with grade III, IV and V diabetic foot ulcers, diabetic ulcers with ischaemic changes, tropic ulcers caused by neuropathy, multiple sclerosis, varicose ulcers, Marjolin’s ulcers were excluded. The study proceeded with comparison of both cases and controls in terms of the wound site and wound area in cm2 and also the pre-dressing status of the wound in terms of discharge, slough and granulation tissue, microbiological evaluation, wound area reduction and mean duration of hospital stay. At the end of each week, the same blind observer measured the surface areas of ulcers. Observations were recorded on the quality of granulation tissue, presence of serous discharge and control of infection.
Statistical Analysis
It was done using Chi–square test. Calibration of the observer was done using the kappa coefficient (0.6), which implied high inter observer reliability.
Results
The 100 patients admitted in the study were divided into two equal and comparable groups. Patients subjected to topical phenytoin dressing were classified as cases and those who underwent normal saline wound dressings were classified as controls. The ages of patients varied from 22 to 75 years. Maximum number of cases (63%) belonged to the age group of 51 to 70 years. The average diabetic foot lesion in our country is 60 years.
The mean age of study group was 48.5±12.49 years and that of control group was 49.74±10.9 years. In both study and control groups, diabetes was more common among males as compared to females. Among them, 74% of the patients were males and 26% were females. On comparing the wound site in each group, it was found that dorsum of foot was commonest in phenytoin group, with 21 cases (42%) and next commonest was heel, with 14 cases (28%). In control group, the commonest site was dorsum of foot with 18 cases (36%) and next commonest was forefoot, with 17 cases (34%).
Among 50 cases in phenytoin group, majority of wounds had surface areas of more than 15cm2 with 29 cases (58%), followed by 12 cases (24%) with 9.1-15cm2. Among 50 cases in control group, majority of the wounds had surface areas of 9.1 to 15 cm2 with 19 cases (38%), followed by 17 cases (34%) with more than 15 cm2. Among phenytoin group, discharge from wound reduced significantly by day 14 and was seen in only 11 cases (22%), whereas in control group, discharge from wound continued to be present in 42 cases (84%).The discharge was present in 15 cases till day 21.This difference was found to be statistically significant (Chi squre = 77.81, p value 0.002,df 1). The discharge from wound among cases and controls with respect to number of days, has been shown in [Table/Fig-1].
Comparison of Granulation Tissue among cases and controls
| No.of Days | Absent | Pale | Good | Absent | Pale | Good |
|---|
| 1 | 50 | 0 | 0 | 50 | 0 | 0 |
| 7 | 9 | 38 | 3 | 37 | 11 | 0 |
| 14 | 0 | 15 | 31 | 0 | 42 | 6 |
| 21 | 0 | 1 | 4 | 0 | 22 | 20 |
| 28 | 0 | 0 | 0 | 0 | 4 | 18 |
| 35 | 0 | 0 | 0 | 0 | 0 | 5 |
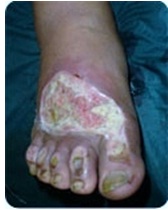
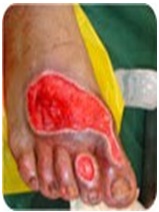
It was observed that the slough from the wound reduced significantly by day 14 and that it was present in only 11cases (22%), among phenytoin group, whereas in conventional group, slough continued to be present in 42 cases (84%).This difference was found to be statistically Significant (Chi–square=16.1, p value<0.005,df1). Among phenytoin group, rate of granulation tissue formation was assessed and pale granulation was seen in 38 cases (76%) and good granulation was seen in 3 cases by day 7, pale granulation was seen in 15 cases (30%) and good granulation was seen in 31 cases (62%) by day 14. In control group, among 50 patients, pale granulation was present in 11 cases (22%) and there was no good granulation till day 7, and good granulation was seen in 6 cases(12%) by day [14]. This difference was found to be statistically significant (Chi–square=17.1, p value <0.005,df 1). Status of wounds before and after phenytoin dressing is shown in [Table/Fig-2 and 3] respectively. The rate of granulation tissue formation among cases and controls has been shown in [Table/Fig-4] respectively.
Organisms Isolated from Wound
| Organisms | Cases | Control |
|---|
| Streptococcus | 2 | 0 |
| Proteus | 2 | 6 |
| Klebsiella | 3 | 2 |
| Citrobacter | 3 | 1 |
| Pseudomonas | 6 | 5 |
| E.coli | 13 | 13 |
| Staphylococcus aureus | 28 | 27 |
| Sterile | 5 | 5 |
The microbiological evaluation of the wounds in both phenytoin and control groups revealed that Staphylococcus aureus was commonest organism. The other organisms isolated from both cases and controls have been shown in the [Table/Fig-4]. The mean duration of hospital stay in phenytoin group was 20.04 (9.141) days, whereas in control group, it was 26.10(5.701) days. This difference was statistically significant (Chi–square = 25.62, p value <0.005,df 1). Phenytoin application showed no complications topically, except mild itching in 5 cases, which was relieved by antihistamines.
Discussion
Diabetic foot ulcer infections account for the largest number of proximate nontraumatic lower extremity amputations [14]. Studies have shown that phenytoin stimulates fibroblast proliferation, decreases collagenase activity, increases epidermal and keratinocyte growth factor receptors, accelerates initial inflammatory responses, induces new vessel formation, speeds the decrease of microbial colonies and improves healing [15,16,10]. It has been well documented in the literature that diabetic foot infections are polymicrobial in nature, but gram positive cocci were the predominant isolates in our study [17,18]. We also isolated good number of aerobic gram negative organisms. There have been similar reports from studies which were done in north India [19]. The isolation rate of aerobic gram negative bacilli was relatively less in many of the western reports [17,18,20].
Phenytoin has been used as an adjuvant for accelerating wound healing since ancient times and number of clinical studies have indicated that phenytoin decreases the bacterial load of wounds [9–11,21,22].Topical phenytoin wasreported to eliminate Staphylococcus aureus, E. coli, Klebsiella spp. and Pseudomonas spp. from wounds within 7-9 days [22].
Clinical studies using topical phenytoin therapy suggest that it may be useful for the treatment of both acute and chronic wounds of various aetiologies, but some studies do not support the use of phenytoin in the treatment of diabetic foot ulcers [23].
Pendse [1] showed that the wound area reduction was greater in the phenytoin group than in controls. Fifty percent of phenytoin-treated wounds had negative cultures by day 7, as compared to 17% among controls. 72.5% phenytoin-treated ulcers had healed completely versus 28.5% controls. Our study showed that in phenytoin group, granulation tissue had appeared in 41(82%) patients, among which pale and good granulation tissues were seen in 38(76%) and 3(6%) patients respectively. In control group, granulation tissue had appeared in 11 cases, among which pale granulation was seen in 11 cases (22%) and no good granulation tissue was observed.
In study conducted by Pai MR [24] the mean percentage reduction of ulcer area was significantly more than that in the control group.(p > 0.05). The mean difference between pretreatment and post treatment values (in cm2) of ulcer area was 6.45 cm2 ±1.53 vs 5.44 cm2±1.49. The phenytoin group showed a slight acceleration of effect as compared to the control group. The mean duration of hospital stay in phenytoin group was 20.04(9.141) days, whereas in control group, it was 26.10 (5.701) days. This difference was statistically significant.
Our observations regarding mean hospital stay were similar to observations of Muthukumarasamy MG [11], who showed that in phenytoin group, mean healing time was 21 days versus 45 days in control group, in which sterile occlusive dressing was used. Most of the patients in both phenytoin and conventional groups had to undergo either split thickness skin graft or delayed primary suturing as a definitive procedure after initial debridement or incision and drainage. No post–operative complications were noted in patients treated with phenytoin.
Conclusion
This study proved that the rate of granulation tissue formation, overall graft survival and patient compliance was better in topical phenytoin dressing group as compared to those in normal saline dressing group. It was also seen that the overall hospital stay was less in the topical phenytoin dressing group. Thus, topical phenytoin moist wound dressing can be considered as a superior option in the management of diabetic ulcers, due to its various actions. But further studies with larger populations, along with other complications, will be needed, for recommending topical phenytoin dressing as major wound dressing among the wide spectrum of treatment modalities available in the management of diabetic ulcers.