Background & Objective: A higher prevalence of periodontal disease among areca nut chewers than non chewers has been demonstrated. Neutrophils, the first line of defence mechanism against microbial infection play an important role in maintaining the periodontal health. In this context our aim was to evaluate the effects of areca nut extracts on phagocytic activity by neutrophils isolated from gingival crevicular washing of healthy subjects and patients with chronic periodontitis.
Material and Methods: Sample size consisted of a total of 60 subjects which were divided into two groups of 30 each. Group I consisted healthy subjects and Group II consisted clinically diagnosed cases of chronic periodontitis. Neutrophils isolated from gingival crevicular washings of both groups were treated with aqueous extracts of ripe areca nut (rANE) and tender areca nut (tANE) and examined for their effect on cellular viability of neutrophils using typan blue exclusion assay. The possible/ ableffects on the phagocytic activity of neutrophils against a periodontal pathogen Aggregatibacter actinomycetemcomitans(ATCC 33384) was determined by using microscopic method.
Results: Both rANE and tANE affected the phagocytic activity by neutrophils in healthy and patients with chronic periodontitis. Ripe areca nut extract has altered the neutrophil functions more than tender areca nut in both the groups. There was no difference seen in the cell viability of neutrophils when treated with rANE and tANE in both the groups (p> 0.05).
Conclusion: Both ripe and tender arecanut extract affected the neutrophil function in healthy and patients with chronic periodontitis. Ripe arecanut extract significantly altered the neutrophils functions more than tender areca nut extract. Thus, alterations in these functions of neutrophils may lead to signs of clinical diseases associated with areca chewing.
Introduction
Periodontal diseases are inflammatory disorders that give rise to tissue damage and loss, as a result of complex interaction between the bacteria and the host immune response [1]. Several bacterial species which reside in biofilms on tooth surfaces, which are referred to as dental plaque, have been closely associated with periodontitis [2].Specific microorganisms, especially gram-negative anaerobic bacteria, which are predominantly seen in periodontal pockets, challenge the host. The most common anaerobic periodontal pathogens which are responsible for periodontal disease are Prevotella intermedia, Fusobacterium nucleatum, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans.
Aggregatibacter actinomycetemcomitans is a facultative gram-negative bacterium which has been associated with severe oral and extra oral infections. A study which was done by Slots, showed that the carrier rate of Aggregatibacter actinomycetemcomitans was 20% for normal juveniles, 36% for normal adults, 50% for patients with adult periodontitis, and 90% for patients with juvenile periodontitis [3].
Periodontal diseases are best considered as the outcome of an imbalance in the host parasite interaction. The narrow balance between periodontal homeostasis and disease depends upon an appropriate qualitative and quantitative response of host defence mechanisms to the infection of the periodontal tissue. Studies which were done on host responses in periodontal diseases have pointed out clearly that the polymorphonuclear leukocytes (PMNs) were the key protective cells, which under normal circumstances, limited the pathology which was caused by periodontal micro- organisms [4].
PMNs enter the gingival crevice in response to chemotactic substances. PMNs or macrophages ingest microorganisms and inert particles by phagocytosis, as an important part of the cellular defense system. It has been shown that the genetic or environmental factors can modulate the initiation and progression of the periodontal disease. Smoking is a potent risk factor in the development of periodontal disease [5,6]. However, the effect of another oral habit, areca nut chewing, on periodontal tissues has not been well documented.
Areca nut chewing is an indigenous habit which is common in habitats of the tropical palm trees which bear these nuts, notably in central, south and southeast Asia, and in some south Pacific islands. It is the fourth most commonly abused substance worldwide and it has been estimated that this habit is being practised by 200–600 million people around the globe, which accounts for 10–20 percent of the world’s population [7]. Areca nut can be chewed alone or in a variety of ways that differ by region, such as fresh and tender areca nut, ripe areca nut and also in the form of quid which consists of areca nut, piper betel leafs and slaked lime, with or without tobacco. Areca nut is composed of various alkaloids and polyphenols. According to Raghven and Baruah, the nut is composed of tannins (11.4-26.0%), alkaloids (0.15-0.67%), fats (1.3-17%), carbohydrates (47.2-84.5%), proteins (4.9-9.3%), non proteins (0.22-1.6%), carotene (5 international units/100gm) and mineral matter (1.0-2.3%). Tannins are of two types, hydrolysable and non hydrolysable. Catechin is a non hydrolysable form of tannin which is present in areca nuts [8].
Areca quid chewing may be associated with a high prevalence of bleeding on probing and high clinical levels of periodontal disease, as there is a likelihood of sub gingival infections with periodontopathogens like Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis [9]. Studies have supported a higher prevalence of periodontal disease among areca chewers than among non-chewers. Moreover, people with a history of areca quid chewing have a higher risk of chronic periodontitis as compared to those who do not have a history of chewing [10]. Apart from the studies which have shown effects of areca nut on periodontal microenvironment and periodontal tissues, few studies have stated that areca nuts affected the immune system.
In this context, our aim was to evaluate the effects of areca nut extracts on phagocytic activity of neutrophils which were isolated from gingival crevicular washings of healthy subjects and patients with chronic periodontitis.
Material and Methods
The study was done in Department of Microbiology, J N Medical College, Belgaum, India, over a period of one year. Sample size consisted of a total of 60 subjects who belonged to either sex and who were above 30 years of age. Written informed consents were obtained from the subjects at the beginning of the study. The study subjects were divided into two groups of 30 each. Group I consisted of healthy subjects and group II consisted of clinically diagnosed cases of chronic periodontitis. Patients with any systemic diseases and neutrophil defects, those who were habitual smokers or areca nut chewers, pregnant females and patients who were on antibiotics for over the past six months were excluded from this study.
Collection of Crevicular Cells
Gingival crevicular cells were obtained from the gingival sulcus in the following manner: The patients were asked to rinse their mouths with clear water for 60 seconds. Prior to collection of crevicular washing, the area was carefully dried with a gentle stream of compressed air. Absorbent cotton rolls were placed in the vestibule. In each case, 15 sequential washings of the gingival crevices were accomplished by using Hank’s balanced salt solution (HBSS) and 2 ml disposable syringes and the washings were collected in 5 μl conventional pipettes [11]. This was then transferred to Eppendrof tubes, and centrifuged at 2000 r.p.m for 10 minutes. Cells were washed twice with HBSS and they were counted by using a Neubauer chamber, with Trypan blue exclusion as an index of cell viability.
Cell Viability Assay
Viability of neutrophils after HBSS (Hank’s buffered salt soln), rANE (ripe areca nut extract), tANE (tender areca nut extract) and catechin treatments was determined by the Trypan blue dye exclusion assay. Treated and control neutrophils were incubated with Trypan blue solution for 3-5 minutes and they were counted on a haemocytometer [12]. The final preparation was studied under a Binocular bright field microscope at 400X magnification. One hundred cells were counted in each preparation and the percentage of cells which excluded Trypan blue stain was counted as a measure of cell viability, which was expressed in percentage.
In vitro Phagocytosis
For phagocytosis, assay freshly isolated neutrophils (107 cells /ml) were incubated with rANE (50μg/ml), tANE (400μg/ml) and catechin (1250μg/ml) for 30 minutes at 37°C on a rotator. Neutrophils which were treated with HBSS only served as controls. Actinobacillus actinomycetemcomitans (ATCC 33384) was combined with treated and control neutrophils in microcentrifuge tubes and the tubes were rotated slowly at 37°C for one hour. Then, from this, smears were made and they were fixed with methanol. They were then stained with Giemsa stain and the microscopically phagocytosed bacteria were counted [12].
Statistical Analysis
Experimental results were expressed as mean + standard deviation (SD). The significance was evaluated by the Mann-Whitney-Wilcoxon test (u-test). For comparison between the groups, one way analysis of variance (ANOVA) test was used and for intra group comparisons, Scheffe’s test was used.
Results
Cell Viability
The percentage of viable cells was calculated per ml. No statistically significant difference noted (p > 0.05) in the cell viability of neutrophils in both Groups I and II when they were treated with HBSS, rANE, tANE and catechin [Table/Fig-1].
Comparison of Cell Viability of Neutrophils when Treated with Different Reagents and Areca Nut Extracts in Group I and Group II
| Reagents/Areca Nut Extracts | Group I n =30 | Group II n =30 | p Value | Inference |
|---|
| HBSS | 85.37+3.62 | 85.27+3.98 | 0.9193 | NS |
| TENDER | 85.17+3.52 | 85.03+4.30 | 0.8959 | NS |
| RIPE | 84.90+3.22 | 84.87+3.64 | 0.9701 | NS |
| CATECHIN | 84.87+3.62 | 84.77+3.75 | 0.9166 | NS |
HBSS : Hank’s balanced salt solution
NS : Not significant
Phagocytic activity
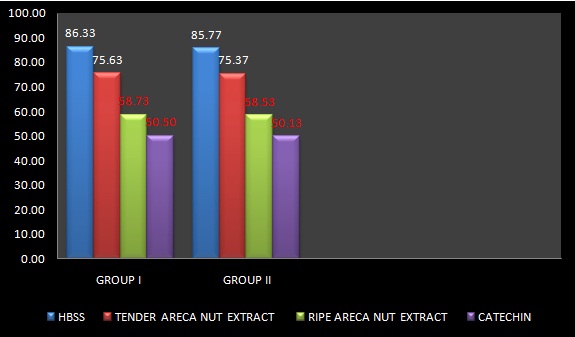
The phagocytosis and adherence of Aggregatibacter actinomycetecomitans by PMNs from crevicular washings of patients with periodontitis and healthy patients were examined microscopically and they were compared. The number of cells with more than 4 ingested or adhered A.actinomycetecomitans was differentiated from viable cells without the bacteria. The highest number of phagocytosed bacteria which was seen in neutrophils which were treated with HBSS, both in Groups I and II was 86.33+5.28 and 85.77+ 6.39 respectively. The lowest number of phagocytosed bactecria which was seen in neutrophils which were treated with catechin, both in Groups I and II was 50.50+ 6.30and 50.13+ 6.36 respectively. When the two groups were compared for their phagocytic activities with the above mentioned reagents and areca nut extracts, no statistically significant difference was found between the groups, with p values of 0.7095, 0.8449, 0.9024 and 0.8232 for HBSS, tANE, rANE and catechin, respectively [Table/Fig-2].
Phagocytosis Assay – Average Number of Aggregatibacter Actinomycetemcomitans Ingested by Neutrophils were Noted as Mean Particle Number (Student’s Unpraired t- Test)
| Reagents/Extracts | Group I n =30 | Group II n =30 | p Value | Inference |
|---|
| HBSS | 86.33 ± 5.28 | 85.77 ± 6.39 | 0.7095 | NS |
| TENDER | 75.63 ± 5.08 | 75.37 ± 5.43 | 0.8449 | NS |
| RIPE | 58.73 ± 6.23 | 58.53 ± 6.36 | 0.9024 | NS |
| CATECHIN | 50.50 ± 6.30 | 50.13 ± 6.36 | 0.8232 | NS |
The mean phagocytosis of the crevicular neutrophils when they were treated with tANE, rANE and Catechin in Group I was 75.63, 58.73 and 50.50 respectively and in Group II, it was 75.37, 58.53 and 50.13 respectively. Statistically significant results were found on intra group comparison (p<0.001). A significant difference was seen in the phagocytic activities of neutrophils in both the groups when they were treated with tANE and rANE [Table/Fig-3].
Intra–group Comparison of Phagocytic Activity within the Group I (Healthy Subjects) and Group II (Patients with Chronic Periodontitis) When Treated with Different Reagents and Areca Nut Extracts
Discussion
Few studies have stated that areca nuts affected the immune system. One of the major players in the inflammatory and immunologic battle ground are neutrophils or PMNs. These cells form the first line of defence mechanism and are present in the superficial periodontal tissues. They can be isolated from the gingival crevicular fluid.
Neutrophils in the gingival crevices can eliminate certain oral microflora by phagocytosis and by killing of microorganisms .Thus, they help in promoting periodontal health by decreasing bacterial colonisation, bacterial growth rate and viability of periodontal pathogens. It has been observed that patients with impaired number of circulating neutrophils and/or functionally abnormal neutrophils were prone to develop periodontal disease.
Thus, the present study examined the effects of areca nut extracts on phagocytic activity of neutrophils which were isolated from gingival crevicular washings of healthy subjects and patients with chronic periodontitis.
The present study comprised of 60 subjects. Group I (healthy subjects) and Group II (patients with chronic periodontitis) comprised of 30 patients each.
After a thorough intra–oral examination, patients were asked to rinse their mouths with water for 60 seconds, so that debris would be removed to some extent. Gingival crevicular washing, which is a diluted form of Gingival Crevicular Fluid (GCF), was collected, to isolate neutrophils, because GCF is a representative of a healthy and periodontal microenvironment. It was observed that cell viability of the neutrophils which were obtained from gingival crevicular washings by Skapski and Lehner technique was between 80-99 % [5,11].
In our study, we employed Trypan blue assay for assessment of cell viability. Dye exclusion tests which are used to assess the cell viability of PMNs are based on the observation that damages to the cell membrane would result in permeability for substances such as Trypan blue, which is not present in undamaged cells. Such tests are common methods which are employed to study cell viability [5].
After exposure to different concentrations of rANE, tANE or catechin in healthy and chronic periodontitis groups, viable number of neutrophils was counted by Trypan blue exclusion assay [12]. The numbers of viable cells were counted by using a microscopic method and we found 84-85% cells remained viable after treatment in both the groups .The viability of the neutrophils did not differ significantly, both in intergroup and intragroup comparisons, thus suggesting that the integrity of the plasma membrane was not affected [Table/Fig-1].
Various methods have been proposed to measure phagocytosis. In our study, we employed the microscopic examination of the neutrophils, after bacteria were added to the suspension of cells, under a prescribed set of conditions. This is the most common quantitative method of assessment for cell associated microorganisms.
In our study, the neutrophils were incubated with Aggregatibacter actinomycetemcomitans ATCC 33384 strains to assess the phagocytic activity of neutrophils under different concentrations of tANE, rANE and catechin. Porphyromonas gingivalis and A.actinomycetemcomitans are leukoaggresive i.e. they produce toxins and other factors which either reduce neutrophil function or kill neutrophils [4]. However, it has been seen that the leukotoxicity of different strains of A.actinomycetemcomitans was not the main determinant that modified its interaction with human neutrophils, since phagocytosis and killing of the leukotoxic strains of A.actinomycetemcomitans could occur before the leukotoxin destroyed the neutrophils [12].
On comparing Group I with Group II, we found no significant difference (p>0.05) in the phagocytic activity of neutrophils when they were treated with tANE, rANE and catechin. Till date, to our knowledge, no studies have been published, which have compared the effects of tANE, rANE and catechin on neutrophil function in healthy subjects and in patients wit h chronic periodontitis. However, in a study which was done by Siguasch et al., to assess the phagocytic activity of neutrophils in healthy subjects and in patients with periodontal disease, they found no difference in the phagocytic activities of healthy subjects and gingivitis and chronic periodontitis groups. Only a reduced activity was seen in aggressive periodontitis and in refractory periodontitis patients [11].
The mean phagocytosis of the crevicular neutrophils when they were treated with tANE, rANE and catechin in Group I was 75.63, 58.73 and 50.50 respectively and in Group II, it was 75.37, 58.03 and 50.13 respectively. Statistically significant results were found on intra group comparison (p<0.001). A significant difference was seen in the phagocytic activity of neutrophils in both the groups when they were treated with tANE and rANE. In both the groups, the phagocytic activity of neutrophils was reduced when they were treated with rANE as compared to tANE. This was consistent with the findings of another study which was done by Hung Sl et al., where they compared the neutrophil function which was obtained from peripheral blood which was treated with various concentrations of tANE, rANE, arecoline and catechin [12]. Another study showed that both rANE and tANE inhibited the phagocytic activity of neutrophils in a dose-dependent manner. Treatment of neutrophils with rANE was more effective than their incubation with tANE [13]. It was observed that the concentrations of arecoline, catechin and total tannin in rANE were higher as compared to those in tANE. Thus, whether additive or synergistic effects among different compounds of areca nuts, which included alkaloids and polyphenols, contributed to the differences between rANE and tANE, remains to be seen [14]. [Table/Fig-2]. Studies have also shown that areca nut extracts reduced early apoptosis, but that they increased the primary necrosis of neutrophils [15].
Conclusion
Earlier, studies had been done to assess the effect of areca nuts on neutrophils from peripheral blood in healthy subjects. But our study was the first of its kind, which assessed and compared the effects of areca nuts on neutrophil function from gingival crevicular washings in healthy subjects and in chronic periodontitis patients. There was a significant reduction in phagocytic activity of neutrophils in healthy subjects and in patients with chronic periodontitis when they were treated with tANE and rANE, thus suggesting that it could contribute to a less efficient elimination of bacteria from the periodontal environment. Thus, it could be one possible mechanism by which areca nut compromised the health of areca nut chewers. Areca nut extract increases Hence, they are advised to have frequent dental check ups and to maintain oral hygiene. Thus, this study has implications in determination of prognosis and treatment planning. Studies have suggested that various alkaloids and polyphenols in areca nuts could affect the neutrophil functions, but the main culprit/s among them, which altered neutrophil functions, still remain/s unexplored.
[1]. Chappell ILC, Reactive oxygen species and antioxidants in inflammatory diseasesJ Clin Periodontol 1977 24:287-96. [Google Scholar]
[2]. Pabst MJ, Pabst KM, Collier JA, Coleman TC, Lemons-Prince ML, Godat MS, Inhibition of neutrophil and monocyte defensive functions by nicotineJ Periodontol 1995 66:1047-55. [Google Scholar]
[3]. Slots J, Reynolds HS, Genco RJ, Actinobacillus actinomycetemcomitans in Human Periodontal Disease: a Cross-Sectional Microbiological InvestigationInfect Immun 1980 29(3):1013-20. [Google Scholar]
[4]. Hart TC, van Dyk SL, Neutrophil defects as risk factors for periodontal diseaseJ Periodontol 1994 65:521-29. [Google Scholar]
[5]. Kenney EB, Kraal JH, Saxe SR, Jones J, The effect of cigarette smoke on Human polymorphonuclear leukocytesJournal Periodontal Research 1977 12:227-34. [Google Scholar]
[6]. Hidalgo FR, smoking and periodontal disease A review of the literatureJ Periodontol 1986 57:617-24. [Google Scholar]
[7]. Lan TY, Chang WC, Tsai YJ, Chuang YL, Lin HS, Tai TY, Areca Nut Chewing and Mortality in an Elderly Cohort StudyAmerican Journal of Epidemiologypublished online on January 4, 2007 [Google Scholar]
[8]. Raghavan V, Baruah HK, Arecanut India’s popular masticatory – History, chemistry and utilizationEconomic Botany 1958 12:315-45. [Google Scholar]
[9]. De Miranda CM, Van Wyk CW, Van der Bijl P, Tygerberg B J, The effect of areca nut on salivary and selected oral microrganismsInternational Dental Journal 1986 46:350-56. [Google Scholar]
[10]. Ling LJ, Hung SL, Tseng SC, Chen YT, Chi LY, Wu KM, Association between betel quid chewing, periodontal status and periodontal pathogensOral Microbial Immunol 2001 16:364-69. [Google Scholar]
[11]. Sigusch B, Klinger G, Holtz H, Suss J, In vitro phagocytosis by crevicular phagocytes in various forms of periodontitisJ Periodontol 1992 63:496-501. [Google Scholar]
[12]. Hung SL, Chen YL, Wan HC, Liu TY, Chen YT, Ling LJ, Effects of areca nut extracts on the functions of human neutrophils in vitroJ Periodontal Research 2000 35:186-93. [Google Scholar]
[13]. Hung SL, Cheng YY, Peng JL, Chang LY, Liu TY, Chen YT, Inhibitory effects of areca nut extracts on phagocytosis of Actinobacillus actinomycetemcomitans ATCC 33384 by neutrophilsJ Periodontol 2005 Mar 76(3):373-9. [Google Scholar]
[14]. Hung SL, Lee YY, Liu TY, Peng JL, Cheng YY, Chen YT, Modulation of phogocytosis, chemotaxis and adhesion of neutrophils by areca nut extractJ Periodontol 2006 77:579-85. [Google Scholar]
[15]. Ho WH, Lee YY, Chang LY, Chen YT, Liu TY, Hung SL, Effects of areca nut extract on the apoptosis pathways in human neutrophilsJournal of Periodontal Research 2010 45(3):412-20. [Google Scholar]