Diabetic Nephropathy is a progressive kidney disease which is caused by angiopathy of capillaries in the kidney glomeruli. It is caused by long standing diabetes mellitus and is a prime indication for dialysis [1]. Once the diabetic patient is on dialysis, the prevalence of cardiac morbidity and the risk for developing ischaemic heart disease de novo is high. Atherogenesis is accelerated in diabetes over and above to that which is found in non-diabetic renal patients [2]. The frequencies of cardiac arrest and sudden death in the dialysis unit are much higher for diabetic patients as compared to those which are seen for non-diabetic patients [3]. Patients with Type -II diabetes who receive dialysis therapy have a poor survival prognosis, mainly due to cardiovascular events. Increased C- Reactive protein (CRP) levels are associated with atherosclerosis and increased risk for cardiovascular events. Local vascular inflammation, which is probably increased in diabetes mellitus due to hyperglycaemic state and oxidative stress [4], is an important factor in the pathogenesis of atherosclerosis [5]. CRP may be a valuable marker of this process [6], while there is also evidence on a causal role of CRP in atherosclerosis [7]. Serum albumin, being a negative acute phase reactant, is also used as a marker of inflammation. The lower albumin levels in diabetic patients may be explained by a state of subclinical chronic inflammation [8]. So, microinflammation is a common finding in haemodialysis patients who have a history of nephropathy with Type-II diabetes mellitus. Identification and proper management of these patients can result in a significant reduction in morbidity and mortality, which was the purpose of undertaking the present study.
Material and Methods
The present study were conducted in the Department of Biochemistry and the Department of Nephrology of Gauhati Medical College (GMC) and Hospital, Guwahati, on 80 diagnosed cases of Type II DM who had end stage renal disease (Nephropathy Stage-5), who were further divided into two groups, Test Group-I (n=40) which included patients who were undergoing haemodialysis and Test Group-II (n=40) which contained patients who were not undergoing haemodialysis. The Control group (n=40) consisted of healthy individuals, who did not have any pathology which was referable to any system, either in the past or present, any recent infection and surgery were not included in this group. Patients with a history of urinary tract infections/pyelonephritis, nephrolithiasis (kidney or bladder stones), trauma, instrumentation, catheterization, foreign bodies in the urinary tract, sickle cell anaemia, cancer (prostate, bladder and kidney), benign prostatic hypertrophy, polycystic kidney disease, familial haemochromatosis, liver disease, a prolonged use of analgesics, any recent infection which was caused by an infectious aetiology and any type of surgery were excluded from the current study. The Study protocol was approved by Research and Ethical committee, GMC, Guwahati. Oral informed consents were obtained from all subjects prior to the start of the study.
By taking all aseptic and antiseptic precautions, 2ml of blood was drawn from the anti cubital veins of the patients. Serum C-reactive protein was estimated by an enzymatic heterogenous, sandwich immunoassay (dry chemistry method) [9] by using an automated analyzer and Serum albumin was measured by BCG method [10]. Glomerular Filtration Rate (GFR) was estimated by Cockcroft-Gault formula [11].
After the biochemical estimations, the results which were obtained were statistically analyzed by using statistical software, SPSS, 16. The results which were obtained were presented as Mean± SD and they were then compared between different groups of study by applying Students ‘t’– test. A probability (p) of less than 0.05 was considered as significant. Correlations were observed on using linear regression analysis.
Results
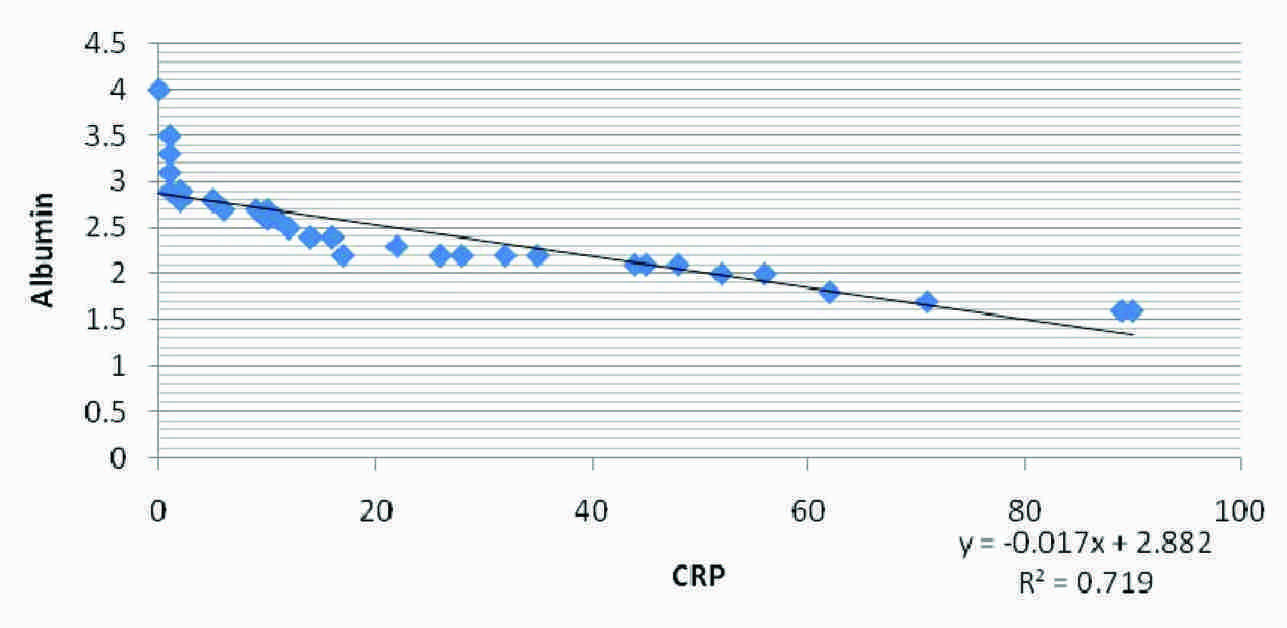
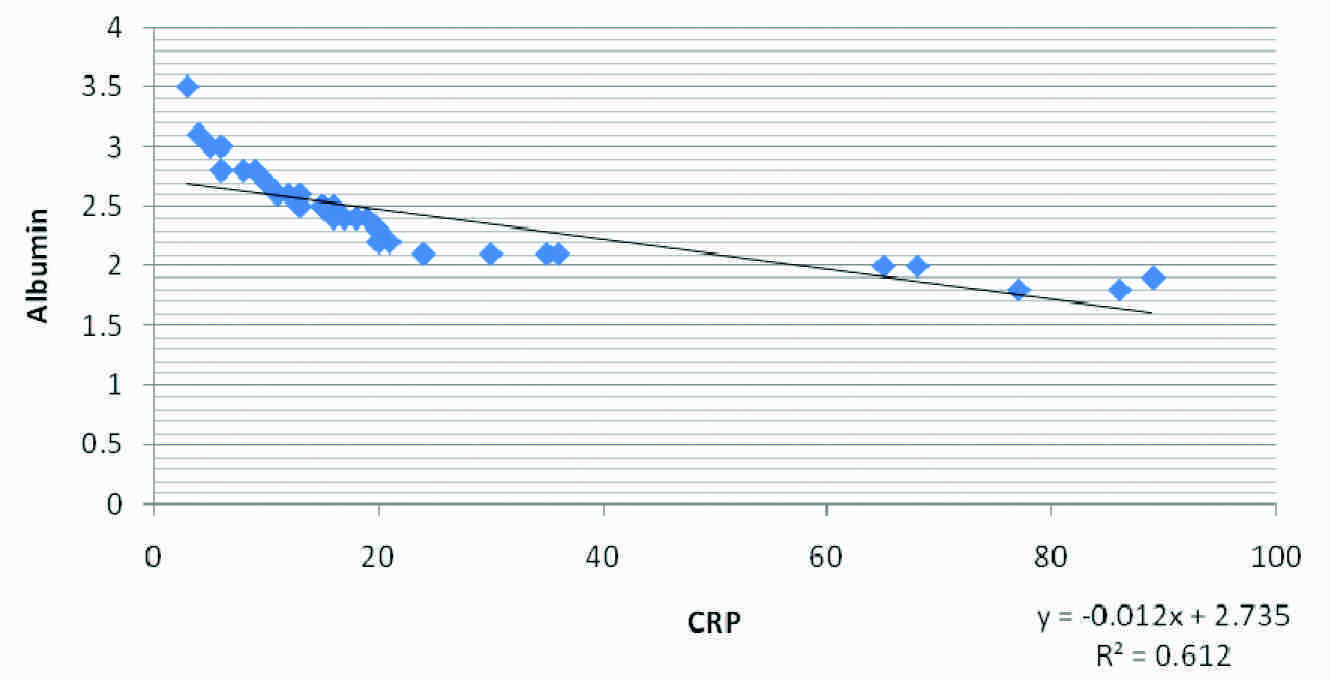
Our results in [Table/Fig-1] show comparison of serum CRP , serum albumin and GFR in Normal Control group and in Test group I and Test group II of end stage renal disease patients (ESRD). Significantly higher values of serum CRP and significantly lower values of serum albumin and G.F.R in Test groups I and II were seen when compared to those of the control subjects (p<0.01). But there were no significant differences among the parameters in Test group I and Test group II. [Table/Fig-2] shows the correlation in Test Group-I and Test Group-II, where a negative correlation between CRP and GFR, a positive correlation in between Albumin and GFR and a negative correlation between CRP and Albumin were seen within the two test groups. Again, [Table/Fig-3 and 4] show the correlational plot of CRP and Albumin in Test Group-I and Test Group-II respectively.
Comparision of CRP, albumin and GFR in the three groups.
| Parameters | Mean ± SD |
|---|
| Normal Control | Test Group - I | Test Group- II |
|---|
| Serum CRP (mg/dl) | 4.125±3.014 | 22.4±24.52**a | 23.425±21.99**b C1 |
| Serum Albumin (gm/dl) | 4.185±0.46 | 2.4975±0.49** a | 2.4375±0.35**b C1 |
| G.F.R (ml/min) | 91.88±31.01 | 9.68±5.5** a | 11.84±6.81** b C1 |
Legend #Values are given as mean ± S.D. Values are statistically significant at **p<0.01 and NS-non significant. Significance compared within the groups are as follows: a. Normal control vs Group I ; b. control vs Group II; c1. Group I vs. Group II non significant.
Correlation coefficients of 3 groups
| Correlation group | Correlation Cofficient (R) |
|---|
| Normal controls | Test Group-I | Test Group-II |
|---|
| CRP and GFR | -0.135 | -0.597 | -0.6231 |
| Albumin and GFR | -0.0519 | 0.904 | 0.946 |
| CRP and Albumin | 0.0232 | -0.848 | -0.78 |
Correlation between Albumin and CRP in test Group I
Correlation between Albumin and CRP in test Group II
Discussion
Renal failure remains one of the most serious complications of diabetes mellitus (DM) and it can ultimately require renal replacement therapy with dialysis or transplantation. The risk of developing end-stage renal disease (ESRD) has been reported to be up to 13-fold higher in men with DM than in those without it [12–13]. Recently, the increased presence of diabetics who undergo dialysis has been shown to be composed predominantly of Type–II diabetics, probably as a consequence of the ageing of the population in industrialized countries and particularly, of the reduction of fatal cardiovascular events [14,15]. This study showed that patients in the test group, who had a history of Type–II diabetes, and were undergoing haemodialysis, had significantly higher values of serum C –reactive protein as compared to those in the control subjects (p<0.01). This finding was comparable to the findings of Dalla Vestra M et al., [15] and Chow FY et al., [16]. The higher CRP level signifies that there is an underlying inflammation which triggers their release from the liver. It is also a very sensitive marker of inflammation. The patients with Type-II DM and overt nephropathy exhibit high levels of diverse acute phase markers of inflammation, which include C-reactive protein (CRP) serum amyloid A fibrinogen, and IL-6 [15–16]. Moreover, the fact that CRP is associated with atherosclerosis and a risk of developing myocardial infarction, is noteworthy [17].
Serum albumin was found to be significantly lowered (p<0.01) in the test groups as compared to its levels in the healthy groups. This finding was comparable to the findings of G Biesenbach et al., [18]. Lower levels of serum albumin may be explained by a state of subclinical chronic inflammation. Reduced serum albumin levels in the patients confirmed that there was an underlying inflammation and serum albumin being a negative phase reactant, was lowered. Also, the lower albumin levels signify poor nutritional status of the test groups. Noe” LJM Cano et al., documented that diabetics who were on haemodialysis as compared to non diabetics, had lower albumin levels [19]. There was a negative correlation between CRP and GFR in Test Group-I with r= –0.597 and Test Group-II, with r=–0.6231 which signified that with a decline in Glomerular filtration rate, the underlying inflammatory process increased and the markers of inflammation like C-reactive protein increased. This finding was comparable to the findings of Shoko Tsuchikura et al., [20]. A positive correlation with r = 0.904 in Test Group-I and with r=0.946 in Test Group-II between Albumin and GFR signified that as the inflammatory process increased, there was a decrease in serum albumin due to its loss into the extravascular space which was caused by increased vascular permeability, its increased consumption by cells locally and its decreased synthesis as a result of its direct inhibition by cytokines [21]. This finding was comparable to the findings of Kaysen GA [22]. The correlation between CRP and Albumin increased with coefficients of correlation (r = -0.848 in Test Group-I and r= -0.78 in Test Group-II), with a negative trend, indicating a definite and a significant degree of an inverse relationship between the [Table/Fig-2]. The poor prognosis of diabetic haemodialysis patients is usually attributed to their co-morbid cardiac and vascular disease conditions [23]. The number of available patients within the time frame and the fact that the study was conducted at only one centre, may be considered as the drawbacks of our study.
Conclusion
Inflammation in chronic kidney disease is the most important factor in the genesis of several complications in renal disease. Underlying inflammatory processes increase in patients of nephropathy with a history of Type-II Diabetes Mellitus, who undergo haemodialysis. Thus, it has been proposed that an early detection of the risk factors of inflammation in diabetic patients who undergo haemodialysis can help us in employing preventive strategies for a better management of the patients and thereby, for improving their survival.
Legend #Values are given as mean ± S.D. Values are statistically significant at **p<0.01 and NS-non significant. Significance compared within the groups are as follows: a. Normal control vs Group I ; b. control vs Group II; c1. Group I vs. Group II non significant.