A Study of Serum Malondialdehyde and Cytokine in Tuberculosis Patients
Rashmi Kulkarni1, Ajit Deshpande2, Ravi Saxena3, Kiran Saxena4
1 Assistant Professor, Department of Biochemistry, Sri Aurobindo Institute of Medical Sciences, Indore, India.
2 Associate Professor, Department of Community Medicine, Sri Aurobindo Institute of Medical Sciences, Indore, India.
3 Professor, Department of Physiology, Chirayu Medical College, Bhopal, India.
4 Professor, Department of Biochemistry, Chirayu Medical College, Bhopal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rashmi Kulkarni, 702, Adarsh Apartment, SAIMS, Indore, India.
Phone: 0731-2729040,9826351549,
E-mail: rashmiak1121@rediffmail.com
Introduction: Tuberculosis (TB) is a common and a deadly infectious disease which is caused by Mycobacterium tuberculi. Inflammatory cytokines play an important role during the course of the disease and they may be responsible for tissue damage which is caused by lipid peroxidation.
Method: The present study was conducted in the Department of Biochemistry and patients were selected from Department of TB and Chest Sri Aurobindo Institute of Medical Sciences and also from Manorama Raje Tuberculosis Hospital, Indore. 32 healthy controls and 35 pulmonary TB patients were compared initially for their serum Malondialdehyde (MDA) and tumour necrosis factor alpha (TNF α) levels. Serum TNF α and MDA levels were correlated.
Result: TNF α and MDA levels in serum were significantly increased (p<0.001) in pulmonary tuberculosis patients as compared to those of controls. Increased Serum TNF α was positively correlated to MDA levels and it was found to be statistically not significant (correlation coefficient r =0.282,p> 0.05 ).
Conclusion: The present study supports the view that there may be a link between lipid peroxidation and cytokine response and relative roles of cytokines and lipid peroxidation in the pathogenesis of tuberculosis
Cytokine, Lipid peroxidation, Tuberculosis
Introdution
Tuberculosis is a common and a deadly infectious disease which is caused by mycobacteria, mainly by Mycobacterium tuberculosis. Globally, there were an estimated 9.27 million incidences of TB [1]. One-third of the world’s current population has been infected by TB, and new infections occur at a rate of one per second. The precise clinical manifestations of tuberculosis (TB) are likely to result from a complex interaction between the host and the pathogen. Cytokines are primarily involved in host response to disease or infection [2]. TNF α is a monocyte-activating cytokine which stimulates antimycobacterial activity and helps in maintaining the integrity of the tuberculous granulomas in which Mycobacterium tuberculosis is contained [3]. TNF α is believed to play multiple roles in the immune and pathological responses in tuberculosis [4]. Serum TNF α measurement might play an important role in the evaluation of the inflammatory phenomena in TB.
During pulmonary inflammation, increased amounts of reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) are produced as a consequence of a phagocytic respiratory burst.[5]. These ROS and RNI induce lipid peroxidation (LP), a general mechanism of tissue damage which is caused by free radicals, that is known to be responsible for cell damage and it may induce many pathological events [6]. Inflammatory cytokines play an important role during the course of the disease and they may be responsible for tissue damage which is caused by lipid peroxidation [7]. Exposure to Mycobacterium leads to cytokine production by macrophages. These cells increase free radical release as a part of bactericidal action, which may, in turn, enhance cytokine secretion, thus creating a positive feedback loop. The present study was carried out to study levels of lipid peroxidation and serum cytokines in tuberculosis patients and to correlate lipid peroxidation and serum cytokine levels.
Material and Methods
Subject: All patients were recruited from the TB and Chest Department of Shri Aurobindo Institute of Medical Sciences and also from Manorama Raje Tuberculosis Hospital, Indore. Thirty-five patients (20 males and 15 females) with pulmonary TB (PTB) and 32 healthy controls (17 males and 15 females) participated in the study. Their mean age was 31.62 ± 6. 37 (range 18-55 years). On their entry into the study, all patients of pulmonary tuberculosis who were found to be new sputum positive, and belonged to category I (two sputum specimens were positive for acid-fast bacilli by direct microscopy or one sputum specimen was positive for culture) were included. No extrapulmonary involvement was found in any of the patient. Those with diabetes mellitus, pregnancy or immunological or autoimmune diseases other than tuberculosis and subjects with a history of smoking were excluded from the study. None of the subjects had any serological evidence of HIV infection. All patients were administered anti-TB therapy according to standard antitubercular regimen. A control group of 32 healthy volunteer subjects (17 women and 15 men) with a mean age of 27.74 ± 7.30 (range18-55 years) was also studied. Consent to participate in the study was obtained from each individual and the study protocol was approved by the institutional and human ethical committees of Sri Aurobindo Institute of Medical Sciences , Indore.
Blood collection: Fasting blood samples (5 ml) were collected by antecubital venipuncture in plain tubes and they were left to clot. They were then centrifuged at 1000 rpm for 10 min. The sera were then aliquoted and stored until they were used for assay of parameters. Blood samples were collected from all controls and from the PTB patients.
Estimation of serum TNF alpha: Serum TNF alpha was estimated by using a commercial immunoassay kit which was available with DIACLONE United Kingdom. The kit was used according to the manufacturer’s instructions. The concentrations of TNF alpha in the samples were determined by using a standard curve and the results were expressed as pg/ml. [Normal range = < 8pg / ml]
Estimation of serum MDA: Estimation of MDA in the serum was done by thiobarbituric acid method of Wilbur KM et al., [8]. Thiobarbituric acid reacts with serum malondialdehyde which is produced by hydrolysis of lipid hydroperoxides to form a pink red colour complex with high absorbance that can be measured spectrophotometrically at 532 nm. (The results were expressed as nmoles/ml.) This complex is usually quantified against MDA standards which are generated from 1, 1, 3, 3 tetraethoxypropane under the same reaction conditions [Normal range = 1-3.42 nmo/ ml].
Statistical Analysis
SPSS, version 10 was used for statistical assessments, to evaluate mean levels of variables between study groups and healthy controls by using Unpaired t-test. Correlations were calculated by Pearson’s correlation coefficients (two-tailed). A p value of ≤ 0.05 was used as a threshold of significance.
Results
There was no significant difference between mean ages of controls and pulmonary tuberculosis patients (p>0.05). The serum levels of TNF alpha and MDA were highly significant in pulmonary tuberculosis patients as compared to those of controls (p<0.001). Among 35 patients, 28 were found to be having increased levels of MDA (ranges 2.90-7.01 nmol/ml) and TNF levels were found to be increased in all patients (range 12-73pg/ml) [Table/Fig-1].
Comparison: Mean Age & Serum Levels of TNF α & MDA in & Pulmonary Tuberculosis Patients Control Group
| Control | Tuberculosis patients | p value |
|---|
| Age | 31.62 ± 6.37 | 29.37 ± 8.2 | p>0.05 |
| Serum MDA nmol/ml | 2.1 ± 0 .82 | 5.04 ± 1.15 | p<0.001 |
| Serum TNF α Pg/ml | 14.59 ± 8.88 | 53.14 ± 19.06 | p<0.001 |
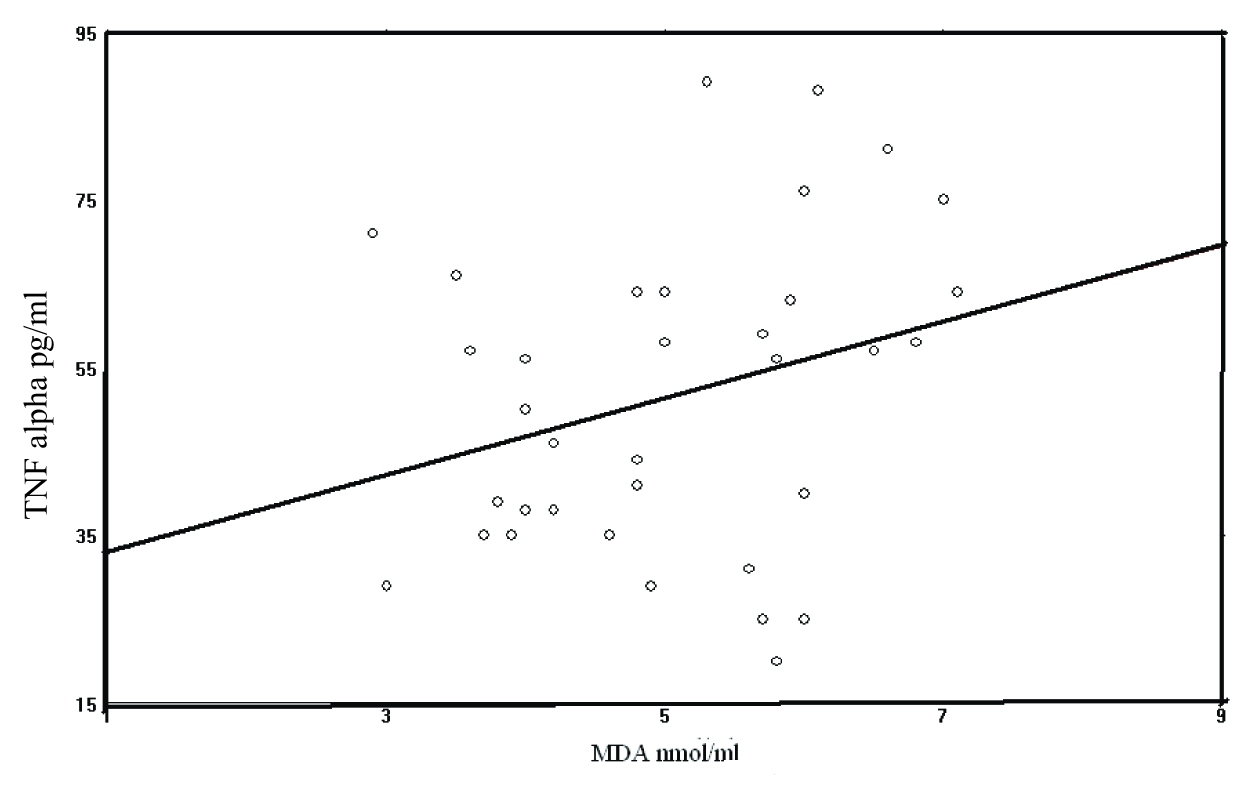
Increased Serum TNF α was positively correlated with MDA levels and this was found to be statistically not significant (r =0.282, p>0.05) [Table/Fig-2].
Correlation between TNF alpha & MDA Pearson’s correlation coefficient, r = 0.282, p>0.05
Disscussion
Pleiotropic cytokine TNF α has been shown to be associated with both protection and pathogenesis in mycobacterial infections [9]. TNF α also appears to be crucial for the formation of M. tuberculosis-constraining granulomas, infection control and elimination of mycobacteria [10]. Previous studies have shown higher serum levels of TNF α in pulmonary TB patients as compared to those in control subjects [11–15].
TNF α is involved in reactive oxygen species (ROS) formation [16]. In response to TNF-α, ROS regulates many important cellular events, which include transcriptional factor activation and cellular proliferation [17]. Cytokines such as interferon-gamma (IFN-γ) and TNF-α, stimulate microbicidal mechanism by inducing the production of reactive oxygen species and nitrogen intermediates [11–18].
Several studies have reported that TNF alpha was an essential factor for host immunity, but overproduction of this cytokine may have serious pathological consequences such as fever, weakness, necrosis and weight loss [19]. Also, a positive correlation was found between increase in serum TNF alpha levels and clinical deterioration in patients with a severe form of TB [20]. Hence, Serum TNF alpha level may be used as a marker of disease activity and inflammation in TB [14, 20, 21].
Inflammatory cytokines play a crucial role during the course of the disease and they may be responsible for tissue damage which is caused by lipid peroxidation [7]. Serum MDA concentration, which is a measure of lipid peroxidation and which reflects the degree of oxidative stress, was significantly higher in patients with tuberculosis as compared to that in healthy controls [22–25]. The MDA levels will be elevated as the anti-TB drugs induce hepatotoxicity and formation of reactive oxygen species (ROS). Those patients with poor antioxidant mechanisms are at a greater risk of toxicity [24–26]. Lipid peroxidation, in turn, leads to the subsequent formation of free fatty acids and arachadonic acid [27]. Free oxygen radicals are general mediators of signal transduction pathways, which are able to induce cytokine production from various cell types [28].
There is a relationship between oxidative stress parameters and inflammatory cytokines. A significant correlation exists between serum TNF-alpha and MDA levels [29]. In the present study, a positive correlation was observed between serum TNF–α and MDA levels, which proposed that increased TNF–α production by activated macrophages could lead to an enhanced production of oxygen radicals and lipid peroxidation. However, the correlation between TNF–α and MDA was not significant, which suggested that inflammation and lipid peroxidation were not parallel occurrences in this study.
The present study supports the fact that there is a link between lipid peroxidation and cytokine response and the relative role of cytokines and lipid peroxidation in the pathogenesis of tuberculosis. Also, serum TNF α and MDA measurements may play an important role in the evaluation of the inflammatory phenomena in TB.
[1]. World Health Organization (WHO). Global Tuberculosis Control; Epidemiology, strategy, financing. 2009 [Google Scholar]
[2]. Kaplan G, Freedman VH, The role of cytokines in the immune response to tuberculosisRes Immunol 1996 147:565-72. [Google Scholar]
[3]. Tufariello JM, Chan J, Flynn JL, Latent tuberculosis, mechanisms of host and bacillus that contribute to persistent infectionLancet Infect. Dis 2003 3:578-90. [Google Scholar]
[4]. Raja A, Immunology of tuberculosisIndian J Med Res 2004 120(4):213-32. [Google Scholar]
[5]. Kwiatkowska S, Piasecka G, Zieba M, Piotrowski W, Nowak D, Increased serum concentrations of conjugated dienes and malondialdehyde in patients with pulmonary tuberculosisRespir Med 1999 93(4):272-76. [Google Scholar]
[6]. Reddy YN, Murthy SV, Krishna DR, Prabhakar MC, Role of free radicals and antioxidants in tuberculosis patientsIndian J Tuberc 2004 51:213-18. [Google Scholar]
[7]. Cem EH, Yusuf TR, Mustafa Cekmen, Serum levels of TNF-a , sIL-2R, IL-6,and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s diseaseMediators of Inflammation 2002 11:87-93. [Google Scholar]
[8]. Wilbur KM, Bernheim F, Shapiro OW, The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acid by various agentsArch Biochem Biophysic 1949 24:305-13. [Google Scholar]
[9]. Bermudez LE, Kaplan G, Recombinant cytokines for controlling mycobacterial infectionsTrends Microbiol 1995 3:22-28. [Google Scholar]
[10]. Bermudez LE, Young LS, TNF alone or in combination with IL-2 but not IFN γ is associated with macrophage killing of M. Avium complexJ Immunol 1988 140:3006-13. [Google Scholar]
[11]. Ameglio FM, Casarini E, Capoluongo P, Mattia GP, Post-treatment changes of six cytokines in active pulmonary tuberculosis, differences between patients with stable or increased fibrosisInt J Tuberc Lung Dis 2005 9(1):98-104. [Google Scholar]
[12]. Kawaguchi H, Ina Y, Ito S, Serum levels of soluble tumor necrosis factor (TNF) receptors in patients with pulmonary tuberculosisKekkaku 1996 71(3):259-65. [Google Scholar]
[13]. Nakaya M, Yoneda T, Yoshikawa M, The evaluation of interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-alpha) level in peripheral blood of patients with active pulmonary tuberculosisKekkaku 1995 70(8):461-66. [Google Scholar]
[14]. Ribeiro-Rodrigues R, Resende-Co T, Johnson JL, Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearanceClin Diagn Lab Immunol 2002 9:818-23. [Google Scholar]
[15]. Rook GA, Taverne WJ, Leveton C, Steele J, The role of gamma-interferon, vitamin D3 metabolites and tumor necrosis factor in the pathogenesis of tuberculosisImmunology 1987 62:229-34. [Google Scholar]
[16]. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M, Reactive oxygen species promote TNF-alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatasesCell 2005 120:649-61. [Google Scholar]
[17]. Fiorenza G, Rateni L, Farroni MA, Bogue C, Dlugovitzky DG, TNF-alpha, TGF-beta and NO relationship in sera from tuberculosis (TB) patients of different severityImmunol Lett 2005 98(1):45-48. [Google Scholar]
[18]. Yuan-Chun Liao, IL-19 Induces Production of IL-6 and TNF and Results in Cell Apoptosis through TNFThe Journal of Immunology 2002 169:4288-4297. [Google Scholar]
[19]. Tramonta JM, Utaipat U, Molloy A, Thalidomide treatment reduces tumor necrosis factor alpha production and enhances weight gain in patients with pulmonary tuberculosisMol med 1995 1:384-97. [Google Scholar]
[20]. Bekker LG, Maartens G, Steyn L, Kaplan G, Selective increase in plasma tumor necrosis factor alpha and concomitant clinical deterioration after initiating therapy in patients with severe tuberculosisJ Infect Dis 1998 178:580-4. [Google Scholar]
[21]. Dahir Ramos de Andrade Júnior, Sânia Alves dos Santos, Isac de Castro, Dahir Ramos de Andrade, Correlation Between Serum Tumor Necrosis Factor Alpha Levels and Clinical Severity of TuberculosisThe Brazilian Journal of Infectious Diseases 2008 12(3):226-33. [Google Scholar]
[22]. Kharakter ZHZ, Skoraia RI, Platonova IL, The ceruloplasmin and lipid peroxidation indices in patients with pulmonary tuberculosisVrach Delo 1989 (10):55-7. [Google Scholar]
[23]. Ghorbanihaghjo A, Rashtchizadeh N, Rohbaninoubar M, Vatankhah A, Rafi A, Oxidative stress in patients with pulmonary tuberculosisSaudi Med J 2006 27(7):1075-7. [Google Scholar]
[24]. Lamsal Madhab, Gautam Narayan, Bhatta Narendra, Dass Bishamber, Bhattacharya Toora Shymal Kumar, Baral Nirmal, Evaluation of lipid peroxidation product, nitrite and antioxidant levels in newly diagnosed and two months follow-up patients with pulmonary tuberculosisSoutheast Asian J Trop Med Public Health 2007 38(4):695-703. [Google Scholar]
[25]. Deveci Figen, Ilhan Nevin, Plasma Malondialdehyde and Serum Trace Element Concentrations in Patients with Active Pulmonary TuberculosisBiological Trace Element Research 2003 95(1):29-38. [Google Scholar]
[26]. Walubo A, Smith PJ, Folb PI, Oxidative stress during antituberculosis therapy in young and elderly patientsBiomed Environ Sci 1995 8:106-13. [Google Scholar]
[27]. Halliwell B, Reactive oxygen species in living systems: source, biochemistry and role in human diseaseAm J Med 1991 91:14-22. [Google Scholar]
[28]. Droge W, Free radicals in the physiological control of cell functionPhysiological Reviews 2002 82(1):47-95. [Google Scholar]
[29]. Makay Balahan, Makay Ozer, Cigdem Yenisey, Gokhan Icoz, Gokhan Ozgen, Erbil Unsal, The Interaction of Oxidative Stress Response with Cytokines in the Thyrotoxic Rat: Is There a Link?Mediators of Inflammation 2009 7 [Google Scholar]