Nimesulide Induced Histopathological Changes in the Vas Deferens of Mice
Thotakura Balaji1, Manickam Subramanian2, Vaithianathan Gnanasundaram3, Sharmila Saran Rajendran4, Hannah Sugirthabai Rajila Rajendran5
1 Professor, Department of Anatomy, Chettinad Hospital & Research Institute, Kelambakam – 603 103, Tamil nadu, India.
2 Assistant Professor, Department of Anatomy, Chettinad Hospital & Research Institute, Kelambakam – 603 103, Tamil nadu, India.
3 Assistant Professor, Department of Anatomy, Chettinad Hospital & Research Institute, Kelambakam – 603 103, Tamil nadu, India.
4 Assistant Professor, Department of Anatomy, Chettinad Hospital & Research Institute, Kelambakam – 603 103, Tamil nadu, India.
5 Associate Professor, Department of Anatomy, Chettinad Hospital & Research Institute, Kelambakam – 603 103, Tamil nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORESPONDING AUTHOR: Dr. Thotakura Balaji, Professor, Department of Anatomy, Chettinad Hospital and Research Institute, Kelambakam – 603 103, Tamil nadu, India.
Phone: 9492776417,
E-mail: drjyothimds@gmail.com
Aim: Nimesulide, a preferential COX-2 inhibitor has 20 times more selectivity towards COX-2 than that of COX-1. COX-2 selective inhibitors cause frequent nephrotoxicity and hepatotoxicity following their usage. This proposes a physiological role of COX-2 in kidney and liver. Not much attention has been focused on the role of COX-2 with respect to reproduction especially in male reproduction, and the available information is scanty.
Aims and Objectives: The present study aims to investigate the adverse effects of nimesulide in the vas deferens thereby indirectly assess the role of COX-2 in male reproductive tract.
Material and Methods: Nimesulide was administered orally and the animals were maintained for different time periods prior to sacrifice.
Results: The vas deferens of nimesulide treated mice showed extensive histopathological changes such as vacoulation and exfoliation of cells in the epithelial layer.
Conclusion: Nimesulide administration leads to cytotoxic effects suggestive of apoptosis in the vas deferens of mice.
Nimesulide, Vas deferens, COX-2, Mice
Introduction
NSAIDs are widely used for the treatment of osteoarthritis, rheumatoid arthritis, dysmenhorrea and pain including headache and post-operative pain [1]. COX-2 inhibitors are considered as novel drugs when compared to other NSAIDs in reducing the inflammation because they target only the COX-2 isozyme [2]. Nimesulide was classified as a preferential COX-2 inhibitor which has 20 times more selectivity towards COX-2 than that of COX-1 [3]. Other specific COX-2 inhibitors includes Rofecoxib and Celecoxib.All these fall under sulfonamide or methyl sulfone group NSAIDs, which are COX-2 selective but are still able to inhibit COX-1 in the blood and in the stomach at clinically prescribed dose and concentration [4]. Nimesulide and diclofenac reduce COX-2 activity at much lower concentrations than they inhibit COX-1 activity in whole blood [5]. COX-2 inhibitors have found to be nephrotoxic during nephrogenesis particularly during last part of pregnancy and early neonatal period leading to fetal renal maldevelopment [6]. Severe hepatotoxic and nephrotoxic effects of COX-2 inhibitors like nimesulide, provesthe fact that COX-2 isozyme is upregulated not only during inflammation.
The role of COX-2 in other organ system is well documented. In kidney certain cells in macula densa contain COX-2. PGs produced by COX-2 may influence renin-angiotensin system [7]. PGE2 is the main product of COX-2 in lung epithelial cells [8]. Very little information is available regarding the role of COX-2 in the reproduction. Immunohistochemical staining for COX-2 in fetal and adult male reproductive tissues have demonstrated that COX-2 is intensely expressed in seminal vesicles and ejaculatory ducts and it might be androgen dependent [9]. In rodents, strong expression of COX-2 is detected in distal vas deferens [10].
The present study focuses on the adverse effects of the drug nimesulide in the vas deferens of mice and indirectly confirms the existence of COX-2 in male reproductive tract of mice.
Material and Methods
Animals and Drug Treatment
A total of 30 adult male albino mice of Swiss strain with body weight 25 ± 2 g and approximately 90 days old were used in this study. All the animals were fed on standard pellet diet (Agro Corporation Private Limited, Bangalore, India). Water was available ad libitum. They were maintained in accordance with the guidelines of National Institute of Nutrition (Indian Council of Medical Research, Hyderabad, India) and the study was approved by Animal Ethical Committee, Annamalai University (Proposal number: 299).
Nimesulide was purchased from Sigma chemicals (St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO) which served as a vehicle. The final concentration of DMSO in water was 0.1%. Nimesulide was administered orally. The animals were divided into the following five groups. Each group comprised of 6 animals. Group 1 served as a control and was administered with 0.1% DMSO. Group 2 and 3 received single dose of nimesulide at 12 mg/kg at 3 hours and 6 hours prior to sacrifice. Group 4 and 5 received nimesulide at 12 mg/kg twice a day for 15 days and 45 days respectively prior to sacrifice. The body weight of all the animals was recorded periodically. At the end of treatment period animals were sacrificed by cervical dislocation, the testis, epididymis, vas deferens and seminal vesicle was removed and the weight was recorded. The vas deferens was further processed for histopathology.
Histopathology
Tissues were dissected and fixed in Bouin’s solution immediately after removal. After adequate fixation they were washed thoroughly in running tap water and dehydrated at 12 hours interval in ascending grades of 50%, 70% and 90% alcohol and finally in isopropyl alcohol. The tissues were cleared in chloroform overnight and embedded in molten paraffin wax. The paraffin blocks were cut at 7μ thickness by using rotary microtome. Serial sections were obtained and mounted on albumin coated glass slides. The sections were dewaxed in xylene, rehydrated in descending grades of alcohol and finally rinsed in distilled water. The tissue sections were stained for nuclei using Harris haematoxylin and blued in tap water. The sections were counter stained for cytoplasm with 1% aqueous eosin excess stain was removed with distilled water further rinsed in 70% alcohol and immersed in xylene. The sections were mounted by using DPX mount, coversliped and viewed under microscope for pathological changes and suitable areas were photomicrographed by using Nikon microscope.
Statistical Analysis
Statistical analysis were performed by using One Way Analysis of Variance (ANOVA) followed by Duncan’s Multiple Range Test (DMRT) by using statistical package of social science (SPSS) version 10.0 for windows. The values are mean ± SD for 6 samples in each group. P-values <0.05 were considered as level of significance.
Results
Body Weight and Organ Weight
The body weight of nimesulide treated animals was similar to that of control animals, no significant decrease or increase in body weight was observed after following nimesulide treatment. The net increase in body weight was similar in control as well as nimesulide treated groups [Table/Fig-1a and b]. No significant changes in weight of the organs namely testis, epididymis, vas deferens and seminal vesicle was noticed in any of the nimesulide treated groups. They were similar to that of control group [Table/Fig-2].
Values are given as mean ± S.D. of six experiments in each group. Values not sharing a common superscript differ significantly at p≤ 0.05 (DMRT)
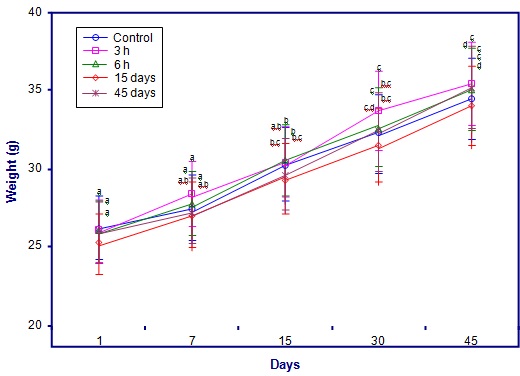
| Body weight of Animal Groups (in grams) |
|---|
| Days | Control | 3 hours | 6 hours | 15 days | 45 days |
|---|
| 1 | 26.2 | 26 | 25.92 | 25.23 | 25.9 |
| 7 | 27.4 | 28.32 | 27.75 | 26.99 | 27.2 |
| 15 | 30.23 | 30.44 | 30.54 | 29.34 | 29.62 |
| 30 | 32.24 | 33.72 | 32.63 | 31.43 | 32.35 |
| 45 | 34.42 | 35.32 | 34.98 | 34 | 35.1 |
Changes in organ weight in control and nimesulide treated mice
| Weight (mg) | Control | 3 hours | 6 hours | 15 days | 45 days |
|---|
| Testis | 121.25 ± 9.23a | 123.47 ± 9.40a | 120.03 ± 9.13a | 122.25 ± 9.30a | 121.14 ± 9.22a |
| Caput epididymis | 25.64 ± 1.95a | 25.43 ± 1.93a | 24.90 ± 1.89a | 25.66 ± 1.95a | 26.32 ± 2.00a |
| Cauda epididymis | 14.90 ± 1.13a | 13.82 ± 1.05a,b | 13.38 ± 1.01b | 14.46 ± 1.10a,b | 14.48 ± 1.13a |
| Vas deferens | 10.99 ± 0.83a | 11.30 ± 0.86a,b | 11.30 ± 0.86b | 11.90 ± 0.90a,b | 11.64 ± 0.88a,b |
| Seminal vesicle | 60.03 ± 4.56a | 59.51 ± 4.53a | 61.02 ± 4.64a | 62.03 ± 4.72a | 61.33 ± 4.67a |
Values are given as mean ± S.D. of six experiments in each group. Values not sharing a common superscript differ significantly at p≤ 0.05 (DMRT)
Histopathology
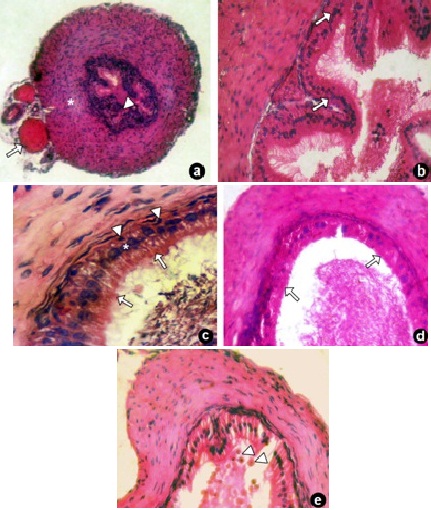
Vas deferens of control group showed normal histological features with an outer serous coat with blood vessels, thick muscular coat and inner mucous layer [Table/Fig-3a]. The mucous layer with its lining epithelium had a definitive folded pattern bearing cilial microvilli [Table/Fig-3b]. Pseudo-stratified epithelium with distinct principal and basal cells were observed [Table/Fig-3c]. No changes were observed in 3 and 6 hours groups. The vas deferens of 15 days nimesulide treated group showed mild disturbances in the epithelium with loss of cilial microvilli [Table/Fig-3d]. 45 days nimesulide treated group showed vacuolation as well as exfoliation of principal cells [Table/Fig-3e].
Photomicrograph of vas deferens of control and nimesulide treated 3a. Photomicrograph of vas deferens in control group showing with intact blood vessels (arrow), muscular (*) and mucosal layer (arrowhead). H & E x 10; 3b. Vas deferens of control group showing mucous folds bearing pseudostratified epithelium (arrow). H & E x 20; 3c. Vas deferens of control group showing principal epithelial cells (*) with intact stereocilia (arrow) and basal cells (arrowheads), a part of lumen with spermatozoa. H & E x 40; 3d. Vas deferens of 15 days nimesulide treated group showing mild vacuolation and loss of streocilia (arrow). H & E x 20; 3e. Vas deferens of 45 days nimesulide treated group showing exfoliation of principal cells (arrowhead). H & E x20
Discussion
Nimesulide and other COX-2 inhibitors are being used to block COX-2 expression and to study its role with respect to female reproduction [11]. With respect to male reproductive organs, COX-2 is detected in the principal epithelial cells of Vas deferens mainly concentrated in the infra-nuclear compartment of the cell. The same pattern of distribution was also observed for COX-1 [12]. Existing reports also state that removal of sperm from lumen of Vas deferens did not alter COX-2 levels. This suggests COX-2 in vas deferens is not derived from sperm [10].
No change in the body and organ weight could be expected after nimesulide administration as suppression of COX-2 might interfere only with free fatty acids especially Arachidonic acid (AA). Even this AA is utilized in minor concentration by COX-2 [13]. Moreover, other class of lipids such as triglycerides and cholesterol is not affected. It is also worth mentioning that nimesulide reduces COX-2 expression but does not completely abolish it [14]. In addition, COX-1 which also utilizes the same substrate as that of COX-2 was not disturbed [15].
Histopathological changes in Vas Deferens after 45 days of nimesulide treatment, proposes a cytotoxic effect due to AA accumulation. Accumulation of AA is observed by following NSAID administration [16]. Our previous studies on fatty acid composition of vas deferens shows a steady rise in AA levels following nimesulide administration. Eicosapentaenoic Acid (EPA) and Docosahexanoic Acid (DHA) which was considered to be protective was found to decrease following nimesulide administration [12]. Suppression of COX-2 with subsequent accumulation of AA leading to apoptosis might be PG mediated. Since increased availability of substrate i.e., AA leads to increased PG production which in turns promotes calcium influx is also quite possible [17]. The possibility of direct toxic effect of nimesulide induced oxidative stress should also be considered. Nimesulide induced oxidative stress also mediated mitochondrial injury through uncoupling of oxidative phosphorylation in hepatocytes [18,19].
To conclude, nimesulide a preferential COX-2 inhibitor influences adverse effect on the vas deferens of mice since COX-2 is thought to be the constitutive isoform in the vas deferens of mice. Nimesulide administration might interfere with reproductive ability of the mice.
[1]. Bejarano PF, Management of inflammatory pain with selective COX-2 inhibitors: Promises and factsCurr. Rev. Pain 1999 3:432-39. [Google Scholar]
[2]. Marnett LJ, Dubois RN, COX-2: A target for colon cancer preventionAnnu. Rev. Pharmacol. Toxicol 2002 42:55-80. [Google Scholar]
[3]. Patrignani P, Panora MR, Sciulli MG, Santini G, Rendo G, Petrono C, Differential inhibition of human prostaglandinendoperoxide synthase-1 and 2 by non-steroidal anti-inflammatory drugsJ. Physiol. Pharmacol 1997 48:623-31. [Google Scholar]
[4]. Panara MR, Padovano R, Scuilli M, Santini G, Renda G, Rotando MT, Pace A, Patrono C, Patrignan P, Effects of nimesulide on constitutive and inducible prostanoid biosynthesis in human beingsClin. Pharmacol. Ther 1998 63:672-81. [Google Scholar]
[5]. Gupta SK, Velpandian T, Mathur P, Sengupta S, Comparative analgesic activity of nimesulide and diclofenac by intramuscular route: Correlation with pharmacokinetic profile of nimesulidePharmacology 1998 56:137-43. [Google Scholar]
[6]. Komhoff M, Wang JL, Chang HF, Langenbach R, Mckanna JA, Harris RC, Cyclooxygenase-2 selective inhibitors impair glomerulogenesis and renal cortical developmentKidney Int 2000 57:414-22. [Google Scholar]
[7]. Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD, Cyclooxygenase-2 is associated with macula densa of rat kidney and increases with salt restrictionJ. Clin. Invest 1994 94:2504-10. [Google Scholar]
[8]. Nakata J, Kondo M, Tamaoki J, Takemiya T, Nohara M, Yogamata K, Augmentation of allergic inflammation in the airways of cyclooxygenase-2 deficient miceRespirology 2005 10:149-56. [Google Scholar]
[9]. Kirschenbaum A, Liotta DR, Yao S, Xin-Hua L, Klausner AP, Unger P, Immunohistochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the human fetal and adult male reproductive tractsJ. Clin. Endocrinol. Metab 2000 85:3436-41. [Google Scholar]
[10]. McKanna JA, Zhang MZ, Wang JL, Cheng HF, Harris RC, Constitutive expression of cyclooxygenase-2 in rat vas deferensAm. J. Physiol. Regul. Integr. Comp. Physiol 1998 275:227-33. [Google Scholar]
[11]. Hull ML, Prentice A, Wang DY, Butt RP, Phillips SC, Smith SK, Nimesulide, a COX-2 inhibitor does not reduce lesion size or number in a nude model of endometriosisHum. Reprod 2005 20:350-58. [Google Scholar]
[12]. Balaji T, Ramanathan M, Menon VP, Localization of cyclooxygenase-2 in mice vas deferens and its effects on fertility upon suppression using nimesulide: a preferential cyclooxygenase-2 inhibitorToxicology 2007 234(1-2):135-44. [Google Scholar]
[13]. Needleman P, Isakson PC, The discovery and function of COX-2J. Rheumatol 1997 24:6-8. [Google Scholar]
[14]. Giuliana F, Warner TD, Ex vivo assay to determine the cyclooxygenase selectivity of non-steroidal drugsBrit. J. Pharmacol 1999 126:1824-30. [Google Scholar]
[15]. Futaki N, Takahashi S, Yokayama M, Arai I, Higuchi S, Otano S, NS-398, a new anti-inflammatory agent, selectivelyinhibits prostaglandin G/H synthase/cyclooxygenase (COX) activity in vitroProstaglandins 1994 47:55-59. [Google Scholar]
[16]. Monjazeb AM, High KP, Koumenis C, Chilton FH, Inhibitors of arachidonic acid metabolism act synergistically to signal apoptosis in neoplastic cell. Prostaglandins LeukotEssent. Fatty Acids 2005 73:463-74. [Google Scholar]
[17]. Cao Y, Pearman AT, Zimmerman GA, McIntyre TM, Prescott SM, Intracellular unesterifiedarachidonic acid signals apoptosisProc. Natl. Acad. Sci. USA 2000 97:11280-85. [Google Scholar]
[18]. Mingatto FE, Rodrigues T, Pigoso AA, Uyemura SA, Curtis C, Santos AC, The critical role of mitochondrial energetic impairment in the toxicity of nimesulide to hepatocytesJ. Pharmacol. Exp. Ther 2002 303:601-7. [Google Scholar]
[19]. Tay VKS, Wang AS, Leow KY, Ong MK, Wong KP, Boelsterli UA, Mitochondrial permeability transition as a source of superoxide anion reduced by the nitroaromaticdurgnimesulide in vitroFree Radical Bio. Med 2005 39:45-59. [Google Scholar]