Erythema multiforme and related disorders comprise a group of mucocutaneous disorders that often compromise the quality of life. The clinical classification of these disorders is variable, thus making definitive diagnosis difficult. Early recognition and prompt management will benefit the patients. This article highlights two such cases of erythema multiforme with detailed literature review on etiopathogenesis, clinical features, and treatment.
Case Report
Case report 1 - A 30 -year- old male patient reported to dental OPD with the complaint of extensive ulcerations in mouth and pain and inability to eat for past 1 week. He gave history of fever and cold two weeks back for which he took Cephalosporin, Paracetamol and Diclofenac injection. Subsequently he developed blisters which later transformed into extensive, irregular ulcerations in the mouth. Pateint visited a dentist for the treatment of ulcers and was put on Novaclox 500mg, Metrogyl 400mg and Diclomol for 5 days and topical application of Chlorhexidine gel, but pain did not alleviate and he was referred to our institution.
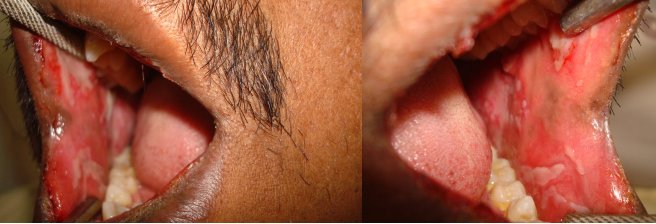
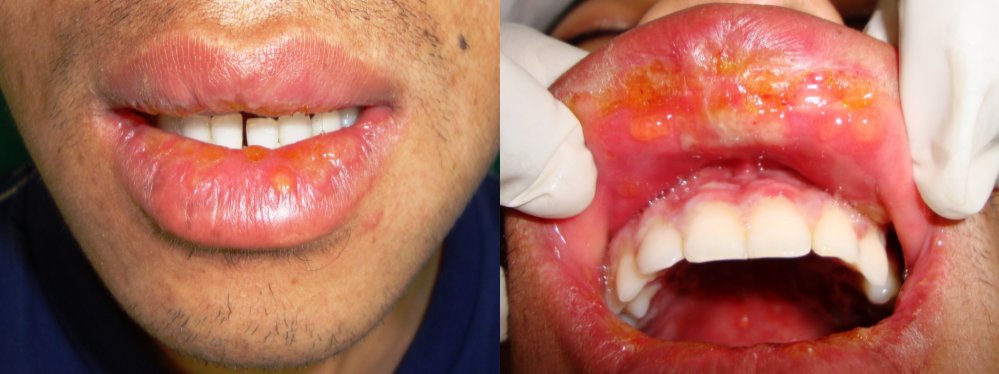
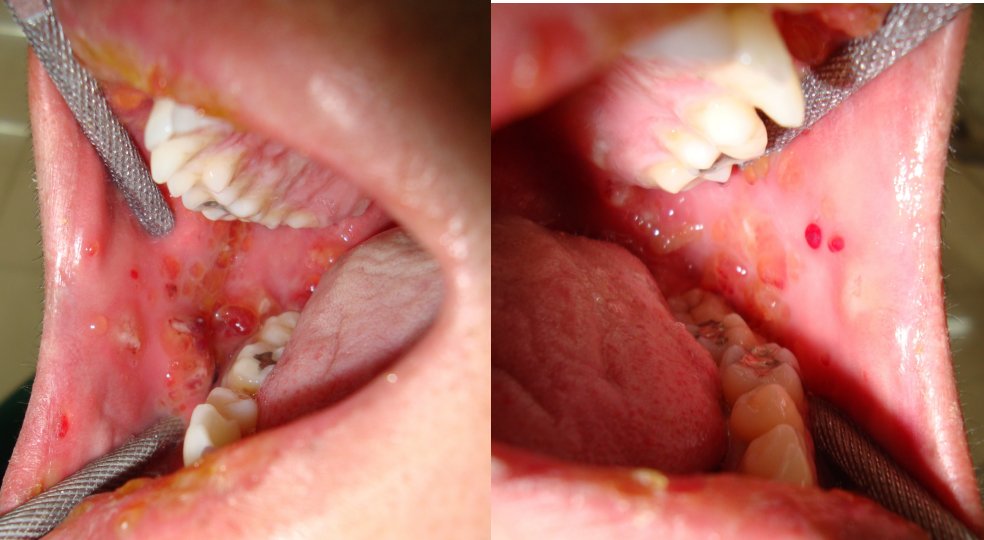
After extra-oral examination, both upper and lower lips showed ulcerations, showing cracking and fissuring with blood encrustation [Table/Fig-1a]. Intra-oral examination showed extensive, irregular ulcerations with sloughing and erythematous borders on buccal mucosa, extending from retrocommissure to retromolar region and extending till the vestibule [Table/Fig-1b). The sudden onset, positive drug history, with above mentioned features lead to diagnosis of oral erythema multiforme. In this case cephalosporin, diclofenac were the causative drugs for the lesion.
Extra oral photograph showing hemorrhagic crusts on the lips

Intra oral photograph showing ulcerations on the right and left buccal mucosa
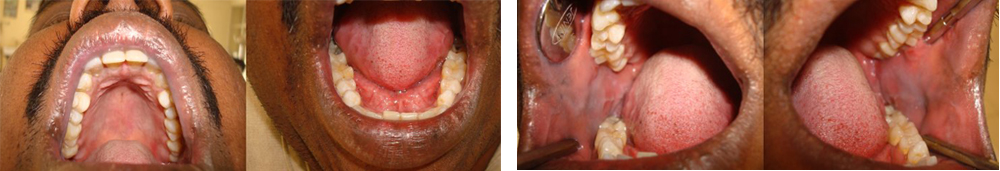
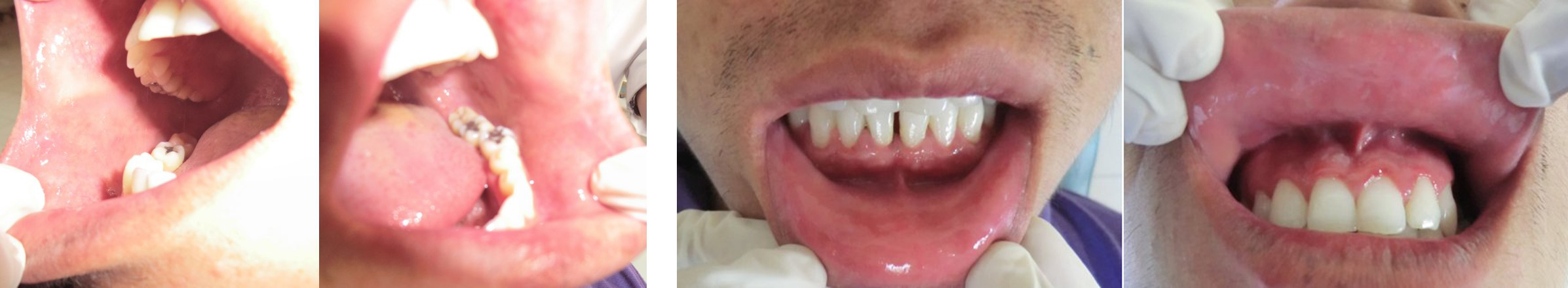
Patient was advised to discontinue the medications prescribed earlier and was treated with systemic corticosteroid (Tab Prednisone 20 mg BID for 3 days followed by tapering dose of 5 mg for 10days), Cap Erythromycin estolate 500 mg BID for 5 days,Tab Metronidazole 400 mg TID for 5 days and topical anaesthetic to aid in oral fluid intake. Healing was noticed on the third day and lesions were completely regressed in 10 days [Table/Fig-2a & 2b].
Follow up photographs after 10 days of the treatment showing complete regression of lesions
Case report 2- A 22- year- old male patient reported to the dental OPD with complaint of ulcers in mouth since 3 days. Patient gave history of having food at a restaurant 3 days back, following which fluid filled blisters started appearing on the buccal mucosa, which increased in number and size. Patient visited physician for the same complaint and topical steroid application was advised.
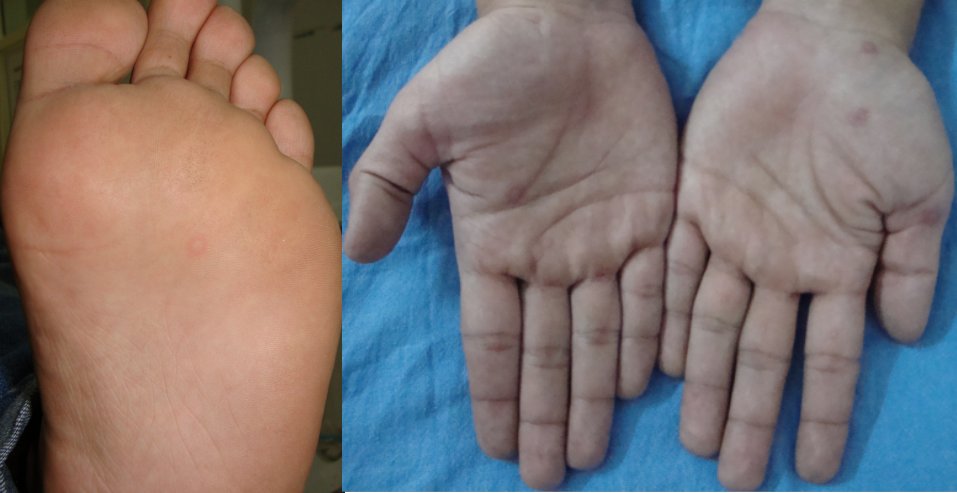
After extra-oral examination, multiple target lesions were seen on the dorsum of hands, palms and sole [Table/Fig-3]. Multiple coalescing bullae with cracking and encrustations were seen on upper and lower lip [Table/Fig-4]. Intra-orally, multiple bullae were noticed measuring about 0.5-0.8 cm in diameter, involving labial mucosa and buccal mucosa [Table/Fig-5]. Slight sloughing was seen on right buccal mucosa and labial mucosa in the area of ruptured bullae, and the intact bullae coalescing to form large irregular boundaries. Nikolsky’s sign was negative. Positive association between the food additive and incidence of lesion and clinical appearance of the lesions lead to diagnosis of erythema multiforme major.
Extra-oral photograph showing target lesions on palm and sole
Photograph showing the fluid filled blisters on the upper and lower lips
Intra- oral photograph showing the fluid filled blisters on the right & left buccal mucosa
An incision biopsy was performed and direct immunofluoresence test showed no immune deposits with IgG, IgA, IgM, and C3. Patient was treated with systemic corticosteroids (Tab Prednisone 20 mg BID with tapering dose of 5 mg for 3 days) Tab Roxithromycin 150 mg BID for 2 days, Tab Acyclovir 400 mg TID for 4 days. Complete regression of lesion was seen after 10 days [Table/Fig-6a & 6b].
Follow-up photographs showing complete regression of the lesion on the right & left buccal mucosa and upper & lower lip
Discussion
Erythema multiforme is a type of reactive mucocutaneous disorder. The reaction pattern appears as a result of allergic host response to antigenic challenge [1]. The oral lesions are accompanied by rapidly rupturing vesicles and bullae leading to diffuse sloughing and ulceration of the whole surface of the skin and mucous membrane.[2,3]. Erythema multiforme can be induced by adverse drug reaction with a frequency of more than 1% [3].
Erythema Multiforme is caused by various insults often from an infectious agents, drugs and food additive [3,4,5]. [Table/Fig- 7 and 8]. In our case 1, the patient had a history of taking multidrugs and case 2 showed lesions due to food additives. Other triggers include benign and malignant tumours, radiotherapy (phenytoin and cranial radiation therapy - EMPACT) [4]. Over 50% of patients have unknown aetiology with stress or emotional factor as the second largest category [2].
Drugs and food additives reported to give rise to EM
| Drugs | Food additives or chemicals |
|---|
| Allopurinol | Benzoates |
| Barbiturates | Nitrobenzene |
| Chemotherapeutic agents | Perfumes |
| Cephalosporins | Terpenes |
| Herbal remedies | |
| Lamotrigine | |
| NSAID’s | |
| Penicillins | |
| Phenytoin | |
| Progesterone | |
| Protease inhibitors | |
| Sulphonamides | |
Micro-organisms association in EM
| Viruses | Bacteria | Fungi and parasites |
|---|
| Herpes viruses: HSV, Epstein Barr virus, Cytomegalo virus, Varicella Zoster virus Adenoviruses Enteroviruses: coxsackie B5, echovirus, HIV Influenza Paravaccinia | Mycoplasma Pneumoniae Corynebacterium diptheriae Neisseria Meningitidis Mycobacterium avium complex Mycobacterium leprae Mycobacterium tuberculosis | Coccidiodomycosis Dermatophytes Histoplasmosis Sporotrichosis Trichimononas Toxoplasma gondii |
The exact pathogenesis is unknown. It has been suggested that EM results from T-cell-mediated immune reaction to the precipitating agent, which lead to a cytotoxic immunological attack on keratinocytes that express non-self antigens, with subsequent to subepithelial and intra-epithelial vesiculation; that leads to widespread blistering and erosions [4].
A better understanding of the molecular and immunologic events underlying HSV-associated EM (HAEM) and their main differences with respect to drug induced EM has been provided by recent studies [4]. It is suggested that disease development begins with HSV infection of epithelial skin cells, and subsequently circulating mononuclear CD34 cells (Langerhans cell precursors). This transports the HSV-DNA fragments to distant skin sites, where an immune mediated epidermal damage occurs due to production of interferon-γ (IFN-γ) [5,6,7].Conversely, in drug-induced EM, the reactive drug metabolites persuade the disease, and tumour necrosis factor alpha (TNF-α) induces keratinocyte apoptosis which is released from keratinocytes, macrophages, and monocytes causing the tissue damage [4,6,7]. A subset of EM patients have been reported to have autoantibodies against desmoplakins I and II and antiepidermal autoantibodies. In addition to a cellular immune response, humoral immune mechanisms may be involved in the pathogenesis of EM-like disease [5,8].
The presentation of EM ranges from self limited, mild form (EM minor) to progressive, and aggressive form like EM major, Steven Johnson syndrome and TEN [1–10] [Table/Fig-9]. Kenneth in 1968 described an inflammatory oral disorder with oral lesions typical of EM. It has been suggested as a third category of EM by many investigators, known as oral EM that are characterized by typical lesions of EM but no target skin lesions [7,9]. Oral EM is chronically recurrent condition, with frequency of episodes varying from every 3 weeks to once yearly. Episodes may be cyclic with duration varying from 10 days to 6 weeks [11]. Our case 1 show lesions limited to oral mucosa and lips, no recurrence was seen on regular follow up for 3 months.
Differential features of erythema multiforme minor, erythema multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis
| Condition | Pattern of skin lesion | Body surface area with epidermal detachment (%) |
|---|
| Erythema multiforme minor | Typical target lesions, raised atypical target lesions, minimal mucous membrane involvement and, when present at only 1 site (most commonly the mouth). Oral lesions; mild to severe erythema, erosions and ulcers. | Less than 10 |
| Erythema multiforme major | Cutaneous lesions and at least 2 mucosal sites (typically oral mucosa) affected. Symmetrically distributed typical target lesions or atypical, raised target lesions or both. Oral lesions usually widespread and severe. | Less than10% |
| SJS | Main difference from EM major is based on the location of lesions and the presence of systemic symptoms Primarily atypical flat target lesions and macules rather than classic target lesions. Generally widespread rather than involving only the acral areas. Multiple mucosal sites involved, with scarring of the mucosal lesions. Prodromal flu-like systemic symptoms also common. | Less than 10% |
| TEN | No typical targets, flat atypical targets; begins with severe mucosal erosions and progresses to diffuse, generalized detachment of the epidermis | Greater than 10 |
Differential diagnosis are to be considered in the lesion confined to oral cavity are herpes, vesicullobullous lesions like pemphigus vulgaris ,bullous pemphigoid. Herpetic lesions are usually smaller and well circumscribed, more common in keratinized mucosa especially in gingiva. Our cases did not have any gingival ulceration. Extensive irregular ulcerations in the lining non keratinized mucosa were seen in our case 1 and case 2 showed mild ulcerations with intact bullae, which were typical of EM and were not feature of herpes infections. Temporal relationship between the drug intake and onset of disease excludes the possibility of any infectious etiology [8,11].
The positive drug and food additive history associated with the onset of ulcerations in our case ruled out the possibility of vesiculobullous lesions like pemphigus vulgaris which may have oral ulcers and skin lesions, but EM have acute onset and does not show desquamative gingivitis [8]. Bullous lichen planus may show similar ulcerations accompanied with Wickham’s straie, which were absent in our cases excluded its diagnosis [10].
There is no specific diagnostic test for EM. Biopsies are advised only in the early vesicular lesions and not in the ulcerated ones as histopathologic appearances are nonspecific [8]. Immunostaining shows intense lymphocytic infiltration at the basement membrane zone and perivascularly, non-specific immune deposits of IgM, C3, and fibrin at these sites [4,9]. Our case 2, showed non specific deposits of IgG, IgM, C3.
Cutaneous patch test may aid in identification of causative agents. To differentiate herpes-associated EMminor/EMmajor from drug-associated EMminor/EMmajor and SJS, the detection of intralesional HSV-DNA via polymerase chain reaction, as well as immunohistochemistry for IFNγ and TNFα, may be useful tests. A rising antibody titre between the acute and convalescent phases of EM major/ SJS may confirm M.pneumonia infection [5] [Table/Fig-9].
No specific treatment is available for EM itself. Triggering agent should be identified and stopped immediately. Summary of management of erythema multiforme is given in [Table/Fig-10]. SJS patients are treated in burn units with porcine xenografts [10].
Summary for Management of Erythema Multiforme and SJS :
| Procedure | Comments |
|---|
| Withdrawal of causative drugs | Withdrawal of likely causative drug therapy can lessen morbidity and mortality [5]. The identification of causative drugs in patients can be difficult Following the “length of exposure” rule can be helpful (i.e., drugs commenced in the last 7-21 days are likely culprits) |
| Supportive care [5,8,9] | Topical analgesics, topical anaesthetics, soothening mouth rinses, bland soft diet, avoidance of spicy food. Broad spectrum antibiotics required for patients with SJS because of consequence of septicaemia may occur. |
| Treatment of precipitating infections | Herpes simplex virus: Antiviral agents may be indicated in HSV infection and a 5-day course of acyclovir 200 mg five times daily at the first sign of lesions, or 400 mg four times daily, or continuous treatment using valacyclovir, 500 mg twice a day [4,9]. Mycoplasmal pneumonia: Empirical use of a macrolide or tetracycline 250mg four times a day for atleast 1week [5] |
| Systemic corticosteroid therapy [4,5,9,11] | Severe recurrent oral EM minor responds to prednisone in doses up to 1mg/kg daily tapered over 2 to 3 weeks |
| Immunosuppressive/Modulating regimens [1,4,5,9,11] Dapsone Azathioprine Mycophenolate mofetil Levamisole Cyclophosphamide Cyclosporine | Dapsone is recommended. An isolated case report has shown the efficacy of dapsone in treating\ recurrent EM Azathioprine has been shown to be consistently effective in producing disease suppression. Mycophenolate mofetil can be tried and it has been shown to be an effective and relatively safe immunosuppressive agent in recurrent EM. Cyclosporine given intermittently may control recurrent EM
|
Conclusion
Erythema mutiforme presents as a reactive ulcerative lesion which has various sources of etiologies and it mimicks other ulcerative lesions. An important step in the management of erythema multiforme is recognition and withdrawal or prevention of contact with the causative agent. As there remains no specific diagnostic test, early diagnosis of disease remains essential to promptly initiate appropriate management and proper follow up. By delivering apt information and educating the patient, oral physicians can play a role in preventing the recurrence of these lesions.
[1]. Sen P, Chua SH, A Case of Recurrent Erythema Multiforme and its Therapeutic ComplicationsAnn Acad Med Singapore 2004 33:793-06. [Google Scholar]
[2]. Shobha BV, Mosby SP, Thanuja R, Erythema Multiforme - A Case ReportJIDA 2010 4(12) [Google Scholar]
[3]. Isik SR, Karakaya G, Erkin G, Kalyonu AF, Multidrug-Induced Erythema MultiformeJournal of Investig Allergol Clin Immunol 2007 17(3):196-98. [Google Scholar]
[4]. Crispian Scully, Jose Bagan, Oral mucosal diseases: Erythema multiformeBritish Journal of Oral and Maxillofacial Surgery 2008 46:90-95. [Google Scholar]
[5]. Al-Johani K A, Stefano F, Porter Stephen R, Erythema multiforme and related disordersOral Surg Oral Med Oral Pathol Oral Radiol Endod 2007 103:642-54. [Google Scholar]
[6]. Michaels B, The Role of Systemic Corticosteroid Therapy in Erythema Multiforme Major and Stevens-Johnson Syndrome A Review of Past and Current OpinionsJournal of clinical and aesthetic dermatology journal 2009 2(3):51-55. [Google Scholar]
[7]. Kohli Parvinderjit S, Kaur Jasbir, Erythema Multiforme-Oral Variant: Case Report and Review of LiteratureIndian J Otolaryngol Head Neck Surg 2011 63:s9-s12. [Google Scholar]
[8]. Fukiwake N, Moroi Y, Urabe K, Norito I, Hashimoto T, Detection of autoantibodies to desmoplakin in a patient with oral erythema multiformeEur j dermatol 2007 17(3):238-04. [Google Scholar]
[9]. Joseph T Isaac, Vargheese Geeta, George Deepu, Drug induced oral erythema multiforme: A rare and less recognized variant of erythema multiformeJournal of Oral and Maxillofacial Pathology 2012 16(1):145-48. [Google Scholar]
[10]. Greenberg MS, Glick M, Ship JA, Burket’s Oral medicine 2008 Eleventh editionBC Decker:57-60. [Google Scholar]
[11]. Lilibeth Ayangco, Roy Rogers, Oral manifestations of erythema multiformeDermatologic clinics 2003 21:195-205. [Google Scholar]