Neurofibromatosis (NF) is an autosomal dominant genetic disorder with a myriad of clinical manifestations . A gastrointestinal involvement which is present in 10 -25% of patients, is usually a systemic manifestation of generalised NF. We are describing a case of NF 1 with chronic constipation, in whom colonoscopy revealed a thickening of the colon wall with narrowing. A mucosal biopsy showed neural hyperplasia .This case emphasizes the value of minimally invasive endoscopic biopsies of GI lesions in NF1, where despite a limited sampling, correlation with clinical and endoscopic features may help in reaching a diagnosis of a neurofibromatous proliferation. We have also discussed the differential diagnosis of gastrointestinal lesions in NF with neural hyperplasia.
Neurofibromatosis, Neural proliferation, GIT, Constipation, Mucosal biopsy
Introduction
Neurofibromatosis (NF) is an autosomal dominant genetic disorder with an incidence of 1:2600-3000 individuals [1]. It has myriad clinical manifestations and a gastrointestinal involvement is present in 10 -25% of patients, as one of the systemic manifestations of generalised NF [1]. The gastrointestinal involvement has a variable phenotypic expression, but it seldom causes clinical problems [2]. This report has highlighted the value of a minimally invasive endoscopic biopsy in the evaluation of gastrointestinal symptoms in a patient with neurofibromatosis.
Case Report
A 21 year male came to the Outpatients Gastroenterology Clinic with complaints of abdominal pain and constipation. On examination, the patient was found to have multiple neurofibromas on the right thigh, one of which was biopsied and histologically diagnosed as a plexiform neurofibroma [Table/Fig-1]. On enquiry, he revealed that his father also had multiple neurofibromas. He therefore fulfilled the criteria for the diagnosis of Neurofibromatosis type I [3]. A complete physical examination revealed no other clinical signs of neurofibromatosis.
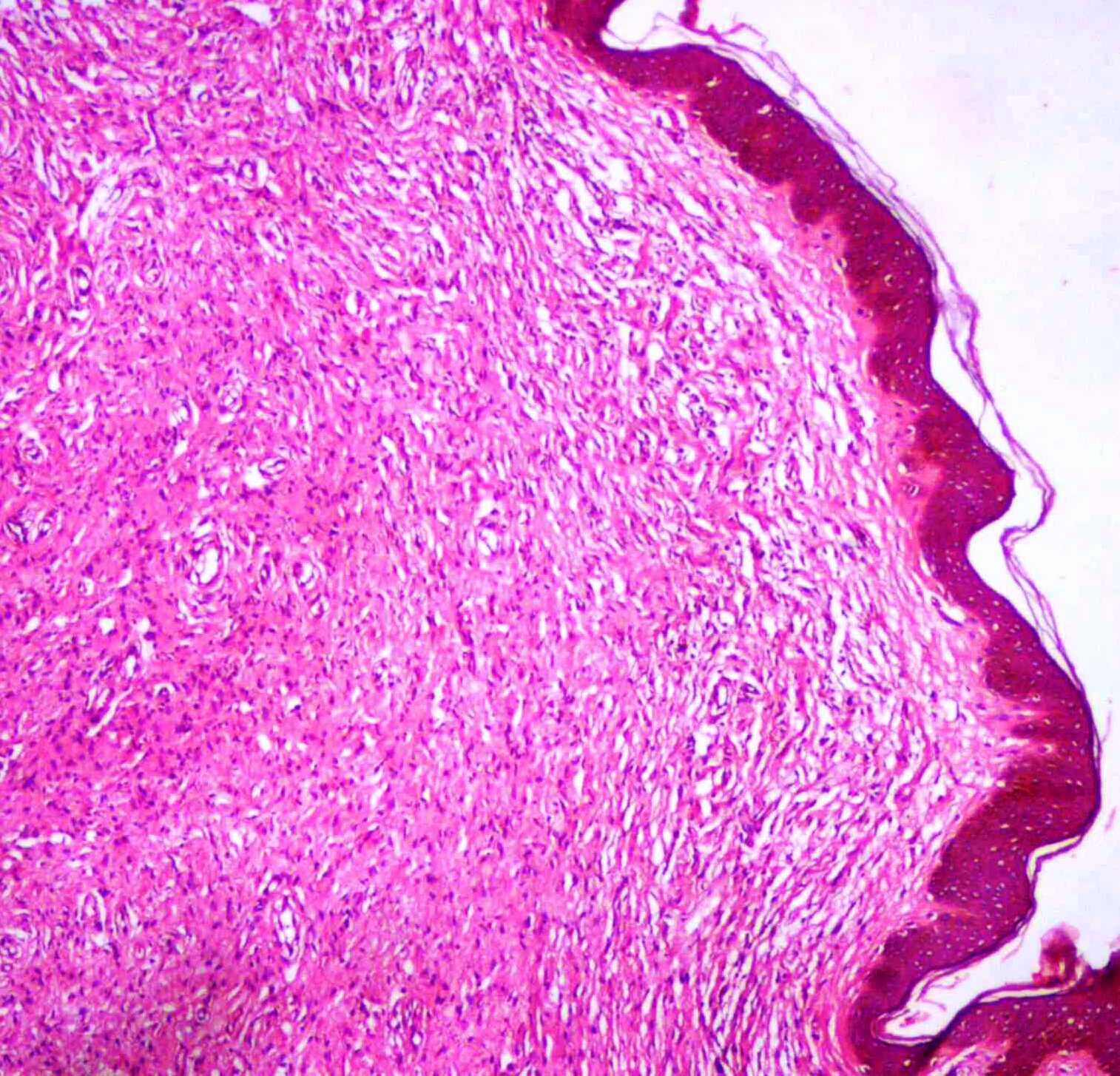
Low power microphotograph from the right nodule biopsy, revealing Schwann cells with elongated wavy nuclei with serpentine configuration with pointed ends arranged in fascicles. (H&E, X100)
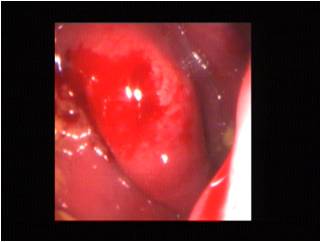
Colonoscopy revealed a nodular to polypoidal thickening in the rectum, which measured 65 mm in right lateral and posterior wall [Table/Fig-2]. The surface mucosa was intact and a mucosal biopsy was taken from this lesion. There were no other lesions in the rest of the bowel mucosa. Thyroid function tests and random blood sugar levels were within normal limits, which ruled out hypothyroidism and diabetes mellitus as causes of chronic constipation. A CT scan of abdomen and pelvis showed an irregular polypoidal thickening of the rectum , which corresponded to that which was visualised on endoscopy. No other tumour was identified in the abdomen on CT scan. A histological study of the colonic biopsy revealed an intact rectal mucosa with tangled fascicles of Schwann-like spindle cells, which widely separated the crypts in the lamina propria. The cells exhibited elongated nuclei with inconspicious nucleoli and abundant eosinophilic cytoplasm. The crypts were lined by normal columnar epithelium [Table/Fig-3]. No ganglion cells were identified despite studying serial sections. Myxoid areas, lymphoid cuffs, fibroblasts, endothelial cells and mast cells were not identified. Immunohistochemistry showed a strong positivity of the spindle cells for S-100 protein [Table/Fig-4]. They were identified as proliferating nerve fibres. On correlating the histopathology with the locations of lesion and endoscopic findings in a background of NF 1, a diagnosis of a neurofibromatous proliferation was offered, keeping in mind the possibility of unsampled ganglion cells, the presence of which would indicate a diagnosis of diffuse ganglioneuromatosis. He was put on symptomatic treatment and was advised follow- up. He has been less symptomatic for the past nine months.
Colonoscopic image – irregular bulge seen beneath the mucosa in the mid–rectum
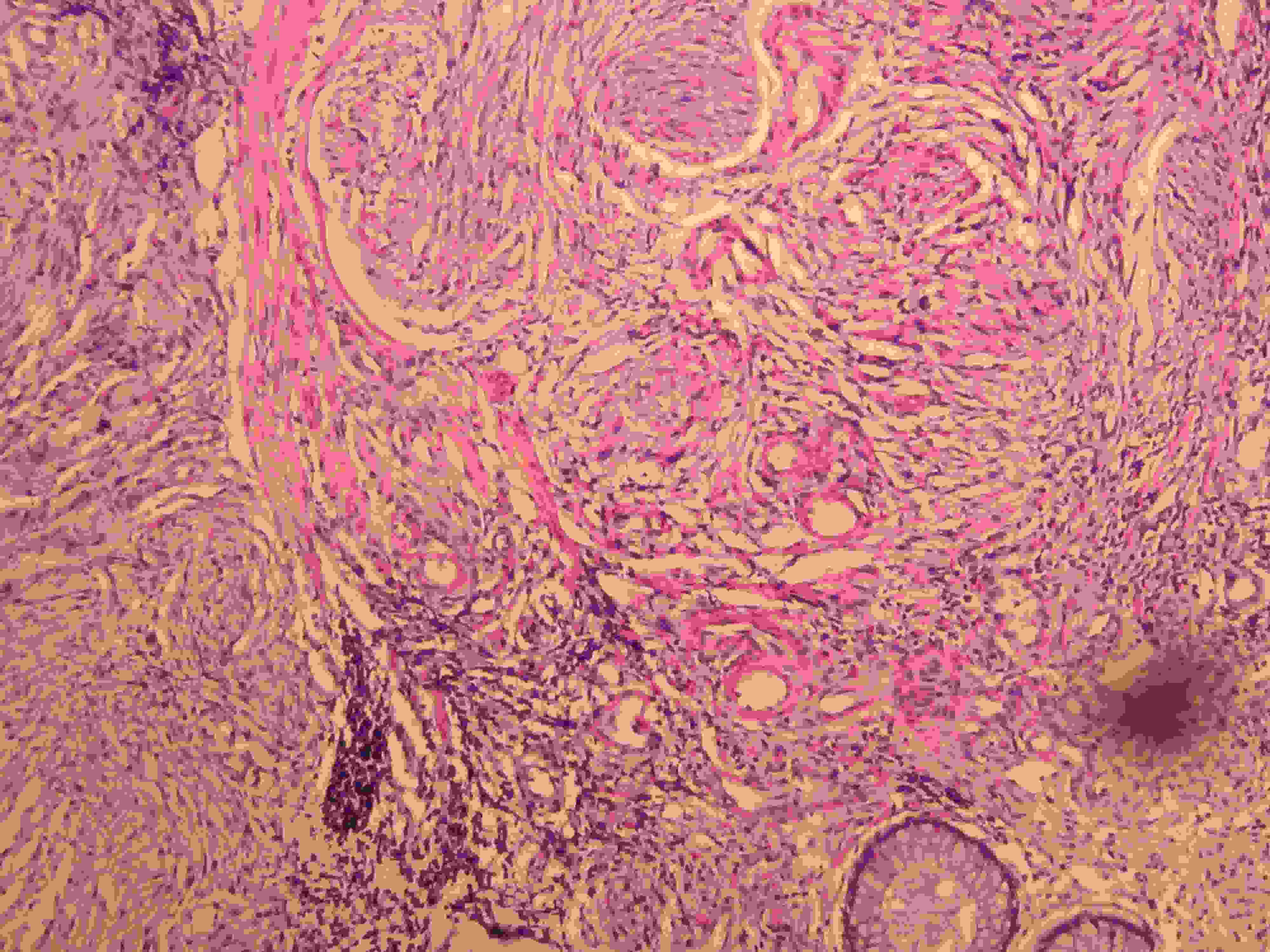
Rectal mucosal glands, tangled fascicles of Schwann –like spindle cells.(H&E,X100)
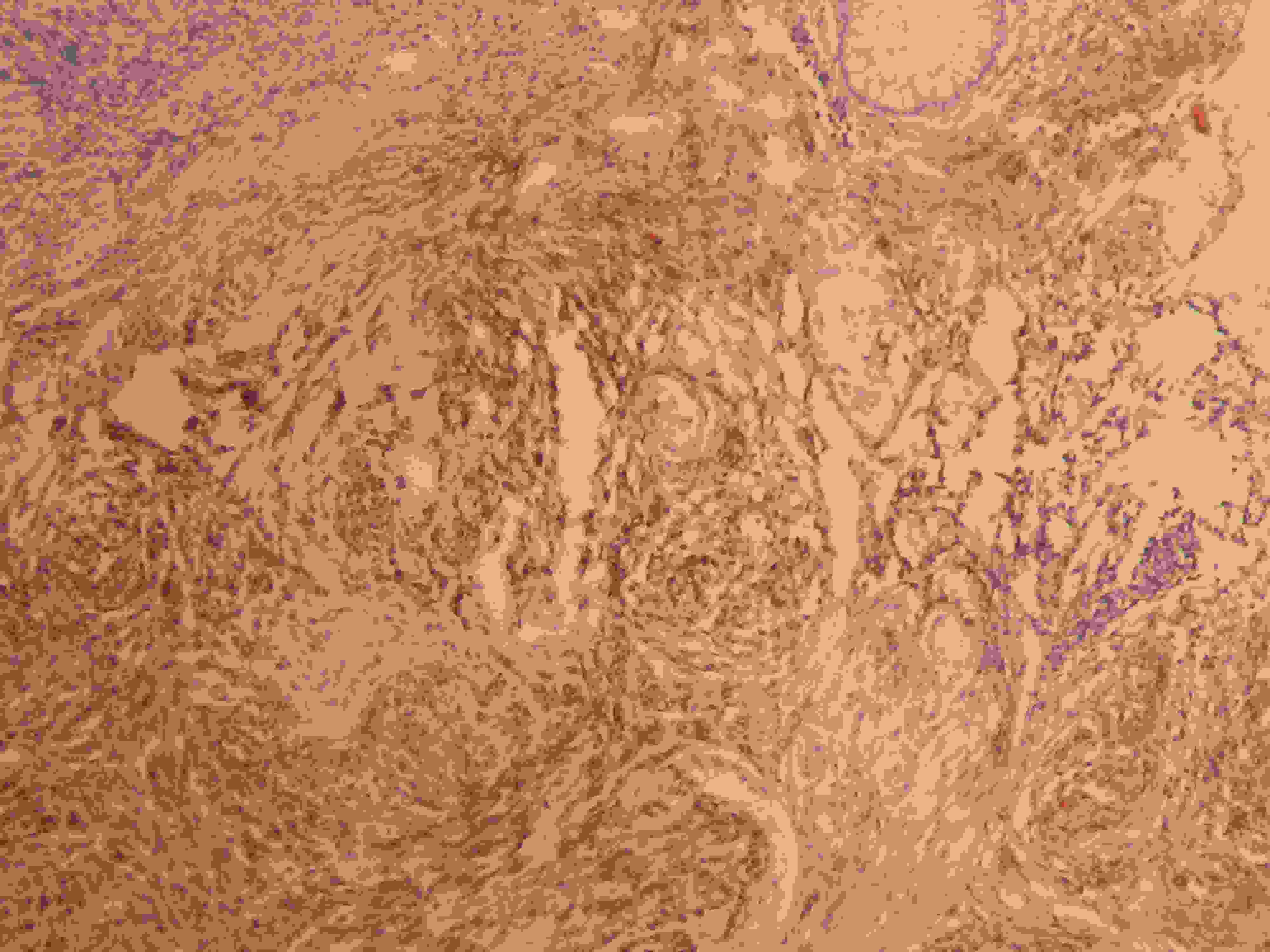
Immunohistochemistry(IHC)- Colonic biopsy-S100 protein positivity of proliferating spindle cells.(IHC, X100)
Discussion
Neurofibromatosis or von Recklinghausen syndrome is an autosomal dominant disease with abnormalities in NF 1 gene which is on chromosome 17 [4].
Involvement of the gastrointestinal tract (GIT) in NF is estimated in 10-25% of cases and it occurs in three forms, hyperplastic lesions of nerve tissue and supporting structures; gastrointestinal stromal tumours (GISTs ) and somatostatin rich carcinoids of the periampullary region [2,5]. The patient in this case had a diffuse colonic lesion which was composed of hyperplastic neural elements.
The differential diagnosis of GI lesions with neural proliferations in NF 1 includes neurofibromas, diffuse ganglioneuromatosis (DGN), schwannomas and malignant peripheral nerve sheath tumours (MPNSTs) [2,6,7]. Neurofibromas develop in small intestine and stomach, but rarely in colon, which are usually solitary, while in NF 1, multiple mucosal and serosal polypoidal nodules develop. Gastrointestinal neurofibromas develop as sporadic isolated lesions or a more diffuse gastrointestinal involvement [7]. Microscopically, the proliferating nerve fibres in NF involve lamina propria, submucosa and /or serosa and no ganglion cells are seen in neurofibroma.
About 25% of individuals who are affected with neurofibromatosis type 1, exhibit multiple intestinal polypoidal neurofibromas or less commonly, ganglioneuromas. Intestinal ganglioneuromas which are overgrowths of nerve tissue may be polypoidal or they may cause diffuse thickenings of the bowel wall [8].
Schwannomas which affect the stomach, colon and rectum are common in NF-1, and they present as polyps. Microscopically, they show S-100 positive Schwann cells which are interspersed with loose myxoid stroma, with peripheral lymphoid cuffs. In the present case, location of lesion, its diffuse morphology on endoscopy and the absence of a myxoid or a lymphoid cuff excluded the diagnosis a Schwannoma. MPNSTs display features of malignancy in nerve sheath tumours, which was not present in this case. Other spindle cell lesions like GISTs, perineuromas and leiomyomas may resemble neural proliferations, but they are usually S-100 negative [7].
DGNs involve colon and rectum, they are poorly demarcated, they vary in size from 1-17 cms and they grow inwards towards the lumen. Endoscopically, they appear as subepithelial masses with nodularity or if they are large , stenosis of the lumen in the affected area [7–9]. DGNs present clinically with constipation, abdominal pain or persistent rectal bleeding. The development of DGNs may be caused by defective tumour suppressor gene signalling of the NF-1 gene, with genetic aberrations in AKT and MTOR signalling transduction pathways [10]. Radiographically, on CT scans and in barium studies, DGNs show segmental mural thickenings of bowel wall [8]. In florid cases of DGN, there is a marked increase in the thickness of submucosal and myenteric plexuses, which is accompanied by proliferation of neural elements like nerve fibres and supporting cells which are arranged as fascicles of bland spindle shaped cells [2,8]. The ganglion cells are variable in number, they are immature or mature, they are scattered in different areas and are seen in the deeper half of the lamina propria [2]. However, at times, when ganglion cells are scanty, the only clue to diagnosis is a solid looking lamina propria, with prominence of spindle cells and separation of the crypts [2]. In this case, only a mucosal biopsy was available, which showed epithelium, lamina propria and minimal superficial submucosa. Given the limited sampling on a single endoscopic biopsy, with a patchy distribution of ganglion cells in DGN, ganglion cells may not have been included in the biopsy. Therefore, in a clinical background of NF 1, where an endoscopic biopsy showed neural hyperplasia, the site and endoscopic features helped us in making a diagnosis of Neurofibromatous hyperplasia , keeping in mind the possibility of unsampled ganglion cells, the presence of which would indicate a diagnosis of diffuse ganglioneuromatosis. In diffuse ganglioneuromatosis, microscopy shows that the lamina propria is hypercellular and that it is composed of bland spindle cells with Schwannian features. Ganglion cells are often isolated and are difficult to identify in some cases [11].
In a review article which was written by Agaimy et al., [12], the authors emphasized that NF 1 patients presented with diffuse neurofibromatous proliferations which expanded the lamina propria, mucosa and submucosa of GIT. This may either take the form of pure neurofibromatosis or it may be admixed with a ganglioneuromatous component. GI manifestations of NF1 were underrecognized by both clinicians and pathologists. However, following the establishment of well-defined diagnostic criteria for neurogenic (Schwann cell) neoplasms in GIT, gastrointestinal manifestations are recognized and they have a substantial impact on their diagnosis.
Panteris et al., [13], states that ganglioneuromatosis refers to extended hyperplasia and hypertrophy of the nerve plexuses and ganglion cells in the mucosa or throughout the intestinal wall. Neurofibromas present as sessile or pedunculated polyps which usually originate from either the Meissner’s plexus in the submucosa or from the Auerbach’s plexus in the muscularis propria.
Jacob et al., [14], in a known case of von Recklinghausen s disease, identified a firm growth in wall of the caecum and ascending colon and diagnosed it as Neurofibromatosis of colon.
In conclusion, neural proliferations in GIT may be seen in the form of different lesions in NF-1. A minimally invasive endoscopic biopsy which is studied in conjunction with clinical, endoscopic and imaging findings helps in making a diagnosis of Neurofibromatous proliferation, which may help in clinical management of the patients of NF1, who present with abdominal complaints.
[1]. Ferner RE, Huson SM, Thomas N, Guidelines for the diagnosis and management of individuals with neurofibromatosis 1J Med Genet 2007 44:81-88. [Google Scholar]
[2]. Fuller CE, Williams GT, Gastrointestinal manifestations of type 1 Neurofibromatosis (von Recklinghausen s disease)Histopathology 1991 19:1-11. [Google Scholar]
[3]. Gutmann DM, Aylsworth A, Carey JC, The diagnostic evaluation and multidisciplinary management of NF 1 and NF2JAMA 1997 278:51-57. [Google Scholar]
[4]. Barker D, Wright E, Nguyen K, Gene for von Recklinghausen neurofibromatosis in the pericentromeric region of chromosome 17Science 1987 236:1100-02. [Google Scholar]
[5]. Bettini R, Ampullary somatostatinomas and jejunal gastrointestinal tumour in a patient with Von Recklinghausen s diseaseWorld J Gastroenterol 2007 13:2761-63. [Google Scholar]
[6]. Donk W, Poyck P, Westenend P, Lesterhuis W, Freid H, Recurrent abdominal complaints caused by a cecal neurofibroma : a case reportWorld J Gastroenterol 2011 17:3953-56. [Google Scholar]
[7]. Mesenchymal tumours. In: Fenoglio-Preiseer CM,Noffsinger A, Stemmermann GN, Lantz PE, Issacson PG edtsGastrointestinal pathology: An atlas and text 2007 3rd ednPhiladelphiaLippincott Williams and Wilkins:1203-60. [Google Scholar]
[8]. Shekitka KM, Sobin LH, Ganglioneuromas of the Gastrointestinal tract- relation to Von Recklinghausen s disease and other multiple tumour syndromesAm J Surg Pathol 1994 18:250-57. [Google Scholar]
[9]. Carter JE, Laurini JA, Isolated intestinal neurofibromatous proliferations in the absence of associated systemic syndromesWorld J Gastroenterol 2008 14:6569-71. [Google Scholar]
[10]. Chan Owen TM, Haghighi Parviz, Hamartomatous polyps of the colon- Ganglioneuromatous , stromal and lipomatousArch Pathol Lab Med 2006 130:1561-66. [Google Scholar]
[11]. Neural tumours . In : Odze RD, Goldblum JR edtsSurgical pathology of GI tract, Liver, Biliary tract and Pancreas 2009 2nd ednSaunders and Elsevier [Google Scholar]
[12]. Agaimy A, Vassos N, Croner RS, Gastrointestinal manifestations of neurofibromatosis type 1 (Recklinghausen s disease): clinicopathological spectrum with pathogenetic considerationsInt J Clin Exp Pathol 2012 5(9):852-62. [Google Scholar]
[13]. Panteris Solitary colonic neurofibroma in a patient with transient segmental colitis: Case reportWorld J of Gastroenterol 2005 11(35):5573-76. [Google Scholar]
[14]. Jacob S, Prabhakar BR, Singh SK, Mammen KJ, Neurofibromatosis of the Colon: An unusual manifestation of Von Recklinghausen s diseases- A case reportIndian J Pathol Microbiol 1998 41(1):113-16. [Google Scholar]