Primary Splenic Tubercular Abscess in an Immunocompromised Patient–Rapid Diagnosis by Line Probe Assay
Savita V. Jadhav1, Chanda R. Vyawahare2, Nabamita Chaudhari3, Neetu S. Gupta4, Nageswari R. Gandham5, Rabinadra N. Misra4
1 Associate Professor, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (DY Patil Vidyapeeth Pune)Pimpri, Pune-18, India.
2 Assistant Professor, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (DY Patil Vidyapeeth Pune)Pimpri, Pune-18, India.
3 Senior Resident, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (DY Patil Vidyapeeth Pune)Pimpri, Pune-18, India.
4 Assistant professor, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (DY Patil Vidyapeeth Pune)Pimpri, Pune-18, India.
5 Professor, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (DY Patil Vidyapeeth Pune)Pimpri, Pune-18, India.
6 HOD and Professor, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (DY Patil Vidyapeeth Pune)Pimpri, Pune-18, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Savita V. Jadhav, Associate Professor, Department of Microbiology, Pad. Dr. D.Y. Patil Medical College, Hospital and Research Center, (D.Y. Patil Vidyapeeth Pune) Pimpri, Pune-18, India.
Phone: 09503994493, Fax: 020- 27420439,
E-mail: patilsv78@gmail.com
Diagnosing extra–pulmonary tuberculosis is a challenge that can confound even the most practiced clinicians as clinical manifestations are vague, non-specific and typical chest radiograph findings may not be evident till late in the disease. Conventional methods for mycobacteriological culture and drug susceptibility testing are slow and cumbersome. Novel technologies for rapid detection of Mycobacterium tuberculosis and its anti–TB drug resistance have therefore become a priority hence with the development of molecular line probe assays are most advanced. Herewith we are reporting a case of splenic tuberculosis in an immunocompromised patient for its rarity and to emphasis the fact that such patients can be diagnosed early for better treatment outcome to enhance the longevity if a health setup possesses all the modern diagnostic services.
Extra–pulmonary tuberculosis, Splenic tubercular abscess, Immunocompromised, Line probe assay
Introduction
In 2009, estimated global annual incidence of Tuberculosis (TB) was 9.4 million cases, out of which 2 million cases were estimated from India. India has one–fifth of the global burden of TB and 40% of Indian population is infected [1]. Tuberculosis in HIV patients enhances the damage to the immune system due to which lymphatic and hematogenous dissemination is more common. In HIV-positive patients, Extra–Pulmonary Tuberculosis (EPTB) accounts for more than 50% of all cases of tuberculosis [2]. The diagnosis of EPTB especially involve deeply located inaccessible area is very difficult. Abdominal tuberculosis is the term used to encompass TB of the gastrointestinal tract, peritoneum, omentum, mesentery and its nodes and other solid intra-abdominal organs such as liver, spleen and pancreas. Immunodeficiency state is an important risk factor for the development of splenic tubercular abscess [1,2,3]. Complications of splenic abscess can be life threatening and include rupture into the peritoneum. Rupture into adjacent organ can occur with the resulting fistulae into the gastrointestinal tract, the pleural space, or lung parenchyma. Splenic tuberculosis co-infection with Human Immunodeficiency Virus (HIV) infection is extremely rare clinical condition [3,4,5]. Line probe Assay (LiPA) is based on nucleic acid amplification technology which allows for rapid detection of Mycobacterium Tuberculosis (Mtb) complex along with genes for rifampicin (RMP) and isoniazide (INH) resistance. Herewith we are reporting here a case of splenic tuberculosis in an immunocompromised patient from tertiary care hospital and highlight the need for rapid diagnostic methods.
Case Report
A 40–years–old female broght to the outpatient department of Gynecology of the Pad. Dr. DY Patil Medical College, Hospital and Research Centre Pimpri–Pune on 15th January 2013, with complaints of dull aching pain in abdomen mainly in the left hypochondrium of one and half month duration. Pain was associated with nausea. She had low grade intermittent fever for 7 days. She had no complaints of cough with expectoration or evening rise of temperature. She underwent hysterectomy 5 months back under spinal anaesthesia. She was at multipara with two pregnancies and two living children (P2L2). There was no past history of diabetes, hypertension or tuberculosis. Two months back, she was admitted and treated for scabies.
The general examination revealed mild pallor and oral thrush (subsequently confirmed by culture as oral Candidiasis). Palpation of abdomen, revealed tenderness of left hypochondrium but no palpable mass. Ascitis was absent. Other systemic examination did not reveal anything significant.
X–ray findings of chest were within normal limits. 2D–ECHO revealed mild mitral regurgitation with mild thickened mitral valve and the ejection fraction as 60%. HIV test by DOT-ELISA and Cassette ELISA was reactive for HIV–1, but HBsAg and VDRL were non–reactive.
The CD4+T Helper cell percentage of CD3 was 10.44, CD8+T Suppressor cells percentage of CD3 was 88.27. Absolute CD8+T Suppressor cell count was 617 cells/c.mm. Ratio of CD4/CD8 was 0.12. total leucocytes count 28200cells/c.mm, the absolute lymphocyte count was 1000 cells/c.mm. 3.6% out of total leukocyte count. Absolute CD3+T Lymphocytes count was 699 cells/c.mm and absolute CD4+T Helper cells count is 73 cells/c.mm.
An Ultra Sonography (USG) of abdomen revealed multiple abdominal lymphadenopathy with many of the nodes showing liquefaction and some showing calcification. A single 2.6x1 cm portal lymph node was also noted. Multiple pre, para aortocaval bilateral iliac lymph nodes were noted, largest size being 3.9x1.7 cm with calcification. The spleen was 11 cm in size with multiple hypoechoic areas suggestive of Multiple splenic micro–abscesses possibly Koch’s aetiology. Other abdominal organs were normal. Finding were strongly suggestive of the splenic tuberculosis.
Splenectomy was done and the splenic tissue was sent to microbiology department for aetiological diagnosis. The specimen from abscess site was processed for direct microscopy, culture and molecular Line Probe Assay (LiPA) for Mycobacterial infection. Zeil-Nelson staining of pus showed the presence of acid fast bacilli. The decontaminated sample was inoculated in MB/BacT/ALERT 3D SYSTEM (BioMerieux France), and growth of acid fast bacilli detected after seven days of incubation. Simultaneously it was also processed for LiPA GenoType MTBDRplus VER 2.0 (Hain Lifesciences GmbH, Nehren, Germany) for rapid detection of Mtb.
Procedure
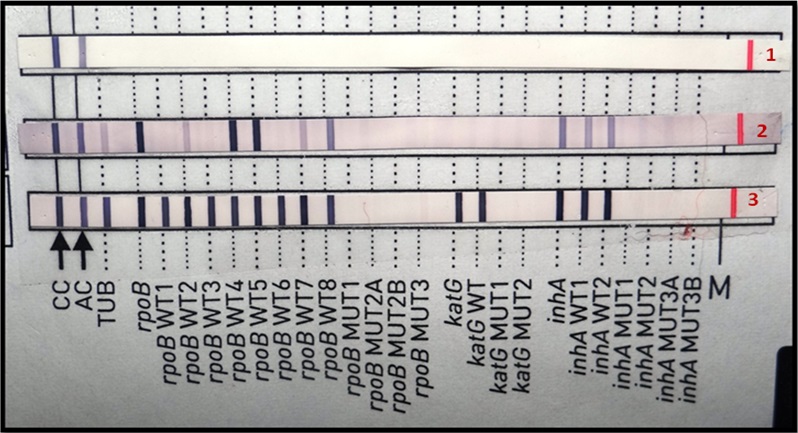
DNA from the decontaminated specimen was extracted using the chemical cell lysis method. DNA extraction kit namely GenoLyse® HAIN Lifesciences France was used for the same. The extracted DNA was then subjected to LiPA for the analysis and diagnosis of TB. PCR was carried out using primers specific for Mycobacterium tuberculosis complex gene loci and the mutations associated with genes for resistance to RMP and INH. An initial denaturation at 95°C for 15min was carried out followed by 30 cycles of denaturation at 950C for 25 sec, annealing at 500C for 40 sec and extension at 700C for 40 sec. Final extension was carried out at 700C for 8 min. The amplified product was then analyzed by “Reverse Hybridization” technique using the DNA strip technology. The strips provided were pre-attached with 27 different probes complementary to MTB complex gene loci and the wild as mutant products of the rpoB, katG and inhA genes that were responsible for imparting resistance to RMP and INH. The banding profile seen was indicative for the presence of Mycobacterium tuberculosis complex and 1st line drug sensitivity/resistance.
Result
The PCR has revealed Mtb complex. The strain was sensitive to RMP and INH [Table/Fig-1]. The patient was treated with AKT 4 (which comprises of Rifampicin, INH, Ethambutol, Pyrazinamide) once daily.
LiPA- Banding profile for splenic tuberculosis. Band -1: Negative control, Band-2: Another sample and Band-3: Patient pus sample
Discussion
Among the differential diagnosis for splenic abscess there can be other infectious causes like S. typhi. Failure to detect the specific cause could be fatal. Splenic tuberculosis is comparatively difficult to diagnose as the clinical presentations are indistinct, non–specific and atypical. Tissue samples for the diagnosis are usually difficult to obtain as it involves invasive procedures. The conventional diagnostic methods have poor chances of recovery and are frequently deferred. Radiological findings cannot reveal the exact aetiology, as it may mimic malignancy or fungal infection and the delay in treatment may end up fatally. Here the rapid microbiological examination by molecular techniques are very necessary to confirm the etilogy in early stage [6,7]. In the present case there was no specific finding; patient came with the problem of pain in left hypochondrium, illness associated with fever of short duration. She was diagnosed as HIV seropositive. In our study the confirmation of mycobacterial infectons was made on the basis of positive acid fast bacilli smear from splenic abscess. Subsequently the molecular technique was used to exclude non–tuberculous Mycobacterial infection and for confirmation of Mtb along with its susceptibility. This was crucial towards starting specific and appropriate ATD. Among the reported cases of splenic tubercular abscess some were associated with HIV co–infection. Hyung Hun Kim et al., (2012) reported primary tubercular abscess of the spleen which was provisionally diagnosed by biochemical markers like positive interferon-gamma release assay and raised adenosine deaminase levels. However detection of such biochemical markers were significant in splenic tuberculosis with ascites [8]. Doris Hillemann et al., (2011) Geneva Switzerland reported a study on the rapid molecular detection of EXPT by automated GeneXpert MTB/RIF system and calculated 77.3% sensitivity and 98.2% specificity. Numerous studies have been assessed the acquiesce of PCR technique for diagnosis of EPTB [9]. Scarparo et al., (2000) compared the performance of E-MTD (Enhanced Mycobacterium tuberculosis direct test- Gen-Probe, San Diego, CA) and the Amplicor Mycobacterium tuberculosis test (Amplicor; Roche Diagnostics Syatem, Inc., Branchburg UK) on clinical samples of tuberculosis and EPTB specimens. They found Amplicor test better than E-MTD as Amplicor test is fully automated [10].
In 2008, WHO published a policy statement on LiPA, recommending its use on smear positive sputum, extra–pulmonary samples and the culture isolates. Following this recommendation, the Central TB Division, Government of India has conceptually approved this technique for diagnosis MDR TB in RNTCP [11,12]. During the past 10 years, several molecular methods have been developed for direct detection, identification and to potentially reduce the diagnostic time from weeks to days. Almost all reported cases of EPTB were diagnosed by radiological finding and histopathological examination of splenic biopsy or splenectomy specimen.
Present case was provisionally diagnosed as spenic tuberculosis. Radiology couldnot pin point the underlying etiology and consequently advanced microbiological methods were needed to confirm the diagnosis. Molecular diagnosis of EPTB by (LiPA) confirmed Mtb complex. The strain was sensitive to rifampicin and isoniazid. The turn it around time to detect MTb complex along with INH, RIF sensitivity by LiPA was < 48 hrs after receiving of specimen in the microbiology laboratory.
Conclusion
High index of clinical suspicion, appropriate and vigilant use of invasive diagnostic methods and rapid confirmation of the diagnosis by sophisticated molecular technological laboratory has become necessary in every health set up to diagnose such cases. In developing countries like India, building up such type of laboratory requires colossal amount of resources. However its role towards reducing the global burden of this disease cannot be over emphasized. Early institution of precise anti-tuberculosis treatment and close clinical monitoring for adverse drug reactions immune reconstitution syndrome are key factors for the successful management of EPTB.
[1]. Swaminathan S, Narendran G, HIV and tuberculosis in IndiaJournal of Biosciences 2008 33(4):527-37. [Google Scholar]
[2]. Sharma SK, Mohan A, Extrapulmonary tuberculosisIndian J Med Res October 2004 120:316-53. [Google Scholar]
[3]. Iscman MD, Tuberculosis in relation to human immunodeficiency virus and acquired immunodeficiency syndrome. In:Iseman M.D. editorAclinicsl guide to tuberculosis 2000 PhiladelphiaLippincott Willams and Wilkins:199-252. [Google Scholar]
[4]. Sharma SK, Mohan A, Sharma A, Challenges in the diagnosis and treatment of military tuberculosisIndian J Med Res May 2012 May 135(5):703-30. [Google Scholar]
[5]. Gonzalez LA, Dronda F, Alonso SM, Clinical significance of splenic tuberculosis in patients infected with human immunodeficiency virusClin Infect Dis 1997 24(6):1248-51. [Google Scholar]
[6]. Sharma SK, Smith-Rohrberg D Tahir, Radiological manifestations of splenic tuberculosis: a 23-patient case series from IndiaIndian Journal of Medical Research 2007 125(5):669-78. [Google Scholar]
[7]. Barone B, Kreuzig PL, Gusmão PM, Case report of lymph nodal, hepatic and splenic tuberculosis in an HIV-positive patientBrazilian Journal of Infectious Disease 2006 10(2) [Google Scholar]
[8]. Hyung HK, Seun JP, Moo IP, Primary tuberculous abscess of the spleen in an immunocompetent patient diagnosed by biochemical markers and radiologic findingsJ Clin Med Res 2012 4(20):149-51. [Google Scholar]
[9]. Doris H, Sabine RG, Catharina B, Rapid molecular detection of extrapulmonar tuberculosis by the automated Genexpert MTB/RIF systemJ Clin microbial 2011 (4994):1202-5. [Google Scholar]
[10]. Scarparo C, Piccoli P, Rigon A, Comparisons of enhanced mycobacterium tuberculosis amplified direct test with cobas Amplicor Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extra–pulmonary specimensJ clin microbial 2000 38:1559-62. [Google Scholar]
[11]. Molecular Line Probe Assays for rapid Screening of patients at risk of Multidrug-resistnat Tuberculosis (MDR-TB): WHO policy statement. 27 June, 2008. Available at http://www.who.int/tb/laboratory/line_probe_assays/en/index.html [Google Scholar]
[12]. Morgan M, Kalantri S, Flores L, A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic rewiew and meta–analysisBMC Infect Dis 2005 5:62 [Google Scholar]