Material and Methods
A cross sectional study was carried out, after obtaining institutional ethical committee clearance, in a 750 bed tertiary care hospital in south India, which caters to patients from Pondicherry and neighboring districts of Tamil Nadu. Informed consents were obtained from patients with MRSA infection, after explaining to them, all aspects of MRSA infection and their participation in a clinical study. A proforma was prepared for recording patient related information. Forty MRSA isolates from clinical materials which were preserved from March, 2004–December, 2009 and 62 new isolates which were recovered during January, 2010 to June, 2011, from various clinical samples such as pus swabs and aspirates, blood, urine, sputum and endotracheal tube aspirates, were included in this study. Consecutive isolates of MRSA from same patients were excluded.
All clinical samples were processed in the laboratory as per standard guidelines. S. aureus isolates were identified by standard laboratory procedures. Disc diffusion test which used a 30μg cefoxitin disc and a 1μg oxacillin disc and oxacillin screen agar test (which contained 6 μg /ml of oxacillin and 4% NaCl) were performed as per Clinical and Laboratory Standards Institute (CLSI) guidelines, to detect MRSA stains [10]. A panel of commonly used anti-Staphylococcal antibiotics (Himedia, Mumbai, India) which comprised of ciprofloxacin (5μg), tetracycline (30μg), gentamicin (10μg), amikacin (30μg), netilmicin (30μg), co-trimoxazole (1.25+23.75 μg), chloramphenicol (30μg), erythromycin (15μg), vancomycin (30μg), linezolid (30μg), clindamycin (5μg), mupirocin (5μg) and rifampicin (5μg) were tested by Kirby Bauer disc diffusion method for susceptibility patterns. All isolates were subjected to MIC testing of vancomycin, which was determined by using HiComb Vancomycin strips (Himedia, Mumbai, India). S. aureus ATCC 25923 and ATCC 43300 were used as controls for antibiotic susceptibility test. The viability of test isolates was maintained by doing periodic subcultures in semisolid nutrient agar.
All data were entered in a Microsoft Excel 2007 spreadsheet and statistical analysis was done by using GraphPad In Stat, version 3.00 (San Diego, CA, USA). Chi-square test was used to compare the two groups. All p values which were < 0.05 were considered to be statistically significant.
Results
Amongst 102 cases, 39 were outpatients and remaining 63 were inpatients and they were predominately males (67.6%). A total of 75 patients (73.52%) were in 16 to 60 years age group. The most common samples were pus swabs from skin and soft tissue lesions and aspirates from superficial and deep abscesses, which constituted 58.8% and 22.5% samples respectively, followed by endotracheal aspirates (3.9%), biopsies (3.9%), blood (2.94%), urine (1.9%) and sputum (1.9%). Eighty one out of 102 patients had pyogenic lesions like superficial and deep abscesses, skin ulcers, postoperative infections, wound infections and other surgical or orthopaedic conditions like cellulitis, fistulas and bone and joint infections. MRSA infections were mainly associated with abscesses (17.64%), especially breast and thigh abscesses, which accounted for 2.9% of total cases each. Among ulcerative lesions, foot ulcers (13.72%) were predominant. In contrast to pyogenic lesions, 12 medical and 9 ICU patients had medical conditions like urinary tract infections, chronic obstructive pulmonary disease, pneumonia, organophosphate poisoning, puerperal sepsis, and endocarditis.
The resistance patterns of MRSA isolates to anti-Staphylococcal drugs in recent years have been compared in [Table/Fig-1]. There was no change in place of isolation of MRSA during these years. Although there was a minor year wise variation in number of cases, patients who were admitted in general surgery and orthopaedic wards were the main source of MRSA at our hospital. MRSA isolates showed highest resistance to cotrimoxazole, ciprofloxacin and gentamicin as compared to the resistance which they showed to amikacin, netilmicin and chloramphenicol in both inpatients and outpatients. High prevalence of ciprofloxacin resistance among outpatients as compared to inpatients was statistically significant (a two tailed p value of 0.0159). Although tetracycline resistance decreased and ciprofloxacin, amikacin, and netilmicin resistance showed increases in recent years, no statistically significant difference was found between MRSA isolates which were recovered during 2004-2009 and 2010-2011. All other drugs showed marginal increases in resistance. All MRSA isolates were sensitive to vancomycin, linezolid, mupirocin and rifampicin.
Resistance pattern of MRSA isolates to anti-staphylococcal drugs in past & recent years
| Antibiotics | 2004-2009 n=40 | 2010-2011 n=62 | P value |
|---|
| Ciprofloxacin | 28 (70%) | 50 (80.6%) | 0.239169 |
| Tetracycline | 20 (50%) | 30 (48.3%) | 1.000000 |
| Gentamicin | 25 (62.5%) | 41 (66.1%) | 0.832279 |
| Amikacin | 7 (17.5%) | 20 (32.2%) | 0.113130 |
| Netilmicin | 1 (2.5%) | 6 (9.6%) | 0.241063 |
| Cotrimoxazole | 31 (77.5%) | 53 (85.4%) | 0.425385 |
| Chloramphenicol | 4 (10%) | 9 (14.5%) | 0.560303 |
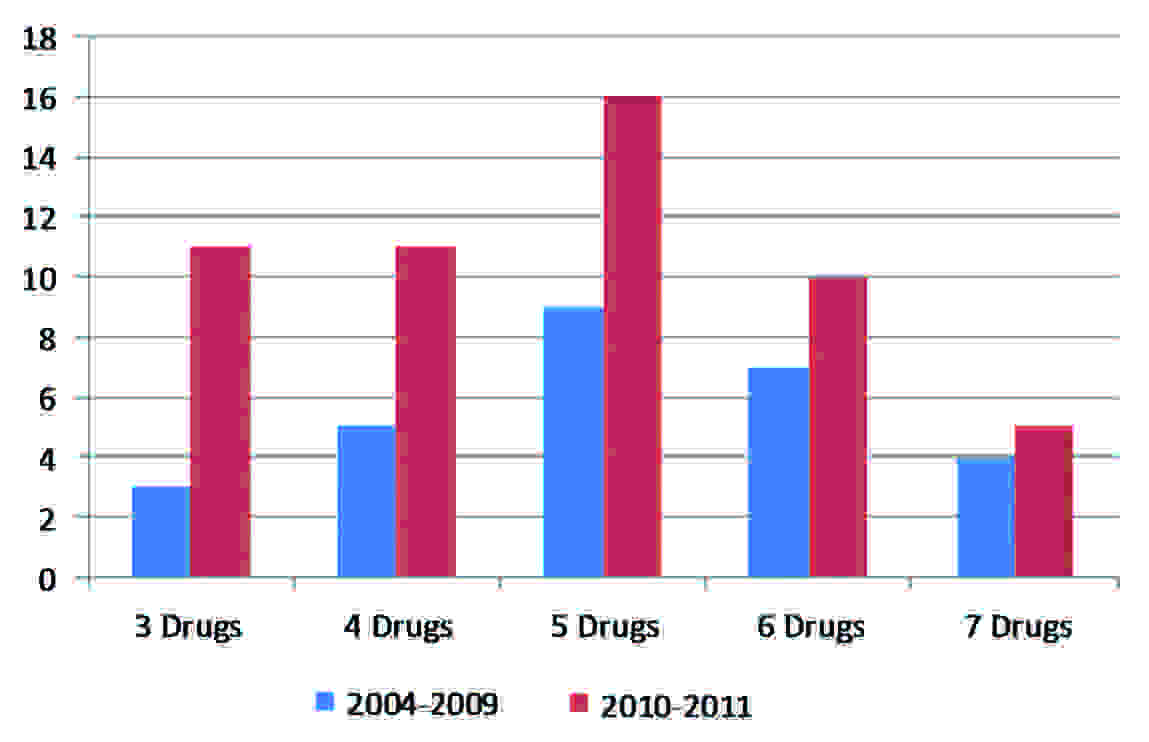
Out of 102 cases, 81 patients (47 inpatients and 34 outpatients) had infections with multi-resistant MRSA (MORSA) (they were resistant to ≥ 3 non beta lactam antibiotics). A majority of MORSA strains had resistance to 3-5 drugs. Furthermore, a multiple non beta lactam antibiotic resistance was seen more in MRSA isolates of 2010-2011 as compared to 2004-2009 isolates [Table/Fig-2]. All strains were sensitive to vancomycin by both disc diffusion and HiComb strip methods. [Table/Fig-3] However, they showed a slight increase in MIC of vancomycin in recent years [Table/Fig-4].
Year wise distribution of multi drug resistance in MRSA strains
Vancomycin MIC of MRSA strains by HiComb strip
| 0.001 μg/ml | 0.01 μg/ml | 0.05 μg/ml | 0.1 μg/ml | 1 μg/ml | 2 μg/ml |
|---|
| OPD (n=39) | 1 | 21 | 9 | 3 | 5 | 0 |
| IPD (n=63) | 5 | 14 | 27 | 12 | 2 | 3 |
Changes in Vancomycin MIC of MRSA strains in past & recent years
| 0.001 μg/ml | 0.01 μg/ml | 0.05 μg/ml | 0.1 μg/ml | 1 μg/ml | 2 μg/ml |
|---|
| 2004-2009 (n=40) | 2 (5%) | 16 (40%) | 13 (32.5%) | 8 (20%) | 0 | 1 (2.5%) |
| 2010-2011 (n=62) | 4 (6.4%) | 19 (30.6%) | 23 (37.1%) | 7 (11.3%) | 7 (11.3%) | 2 (3.2%) |
Discussion
MRSA is a major cause of nosocomial outbreaks and serious infections, which causes increased mortality and morbidity. Skin and soft tissue infections, wound infections, burns, and ulcers, pressure sores, lower respiratory and urinary tract infections, septicaemia and infections associated with invasive devices are most frequently reported [3]. Hospital workers with dermatitis or with inadequate hand washing or asepsis, burns patients and long term care facility patients are the main sources of MRSA in hospitals. In some countries with high prevalences of MRSA, after admission screening, potential MRSA carriers are isolated in single rooms or they are nursed together (cohort) in a room or a bay by maintaining contact precautions, until their screening reports turn out to be negative. In case of positive screening results, isolation measures are employed, along with decolonisation regimens. The entry of visitors, especially children and persons with compromised immunity, should be restricted. In present study, we found a higher proportion of MRSA cases among surgical patients. This may be related to the poor environmental cleaning, operation theatre surveillance and infection control measures of hospitals in Indian setup. According to one study, 80% MRSA isolates were isolated from surgical units, due to higher numbers of post–operative wound infections [6]. Not only infections, even asymptomatic colonisations were also reported to be significantly high in surgical (18%) and orthopaedic (34%), patients as compared to medical unit (1%) patients [11]. Consistent with suppurative nature of Staphylococcal infections, we found that highest number of MRSA isolates was obtained from pus. Although some investigators have reported no significant correlation with gender in MRSA infection [12], in Southern India, it was found more frequently in male patients [13], which was also observed in our study.
This study analysed antibiotic susceptibility pattern of 102 isolates against a panel of non-beta lactam antibiotics and found three major developments in antibiotic resistance over seven years. Firstly, increased resistance to ciprofloxacin in outpatients was statistically significant. Secondly, except tetracycline, all other drugs, specifically, ciprofloxacin, amikacin and netilmicin, showed a greater increase in resistance from 2004-2009 to 2010-2011. Last, but not the least, we found a slow emergence of a reduced susceptibility to vancomycin, as was indicated by an increase in its MIC in recent years. This may reflect slow development of bacterial antimicrobial tolerance in response to increasing use of antibiotics in recent years, especially fluoroquinolones, in outpatients. There are evidences of a significant decrease in fluoroquinolone-resistant MRSA over years, on reduction of fluoroquinolone use [14]. As per recent studies, the resistance pattern of MRSA in India has shown a variable pattern. There are inadequate countrywide studies which have been done on changes in antibiotic resistance on a long term basis in India. Verma et al., has reported a rapid increase in MRSA prevalence, from 12% to 80.89%, over seven years, in a tertiary care centre at Indore [8]. As per current Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group’s report, the prevalence of MRSA varies from 22% to 68% in Indian hospitals, which is clearly higher than previous estimates [9]. According to INSAR report, 79.3%, 70.8%, 58.3%, 55.6% and 46.6% MRSA isolates showed resistance to ciprofloxacin, erythromycin, gentamicin, co-trimoxazole and clindamycin respectively. Thind et al., found only 12.5% isolates to be resistant to tetracycline and 37% isolates to be resistant to cotrimoxazole, while they were fully sensitive to chloramphenicol, ciprofloxacin, gentamicin, amikacin, netilmicin and rifampicin [15].In contrast to this, Anupurba et al., reported a higher resistance of 84.1%, 47.5%, 89.7% and 60.5% against ciprofloxacin, netilmicin, gentamicin and amikacin respectively [16]. The resistance which was detected in other studies was intermediate of these two reports [11, 17–19].
All studies reported universal sensitivity of MRSA to vancomycin, linezolid and mupirocin. However, from northern India, Deep et al., had reported linezolid resistance in 9% MRSA [20]. Reports on reduced vancomycin sensitivities with borderline MICs are not uncommon nowadays [21, 22]. Although, we found that all MRSA strains had MICs in sensitive range, [Table/Fig-3] more number of MRSA isolates from 2010-2011 showed higher MICs for vancomycin as compared to 2004-2009 isolates [Table/Fig-4]. This indicated emergence of a decreased sensitivity to vancomycin, which could develop into a low level resistance in future.
MRSA strains with three or more non-beta lactam drug resistances have been described as multi-resistant oxacillin resistant S. aureus (MORSA) and they have been correlated with treatment failure [23]. We found that 79% MRSA isolates were multi drug resistant. Furthermore, multi-drug resistant strains were more common in inpatients (58%) than in outpatients (42%) and their proportion has increased from 34.5% to 65.4% in 2010-2011 as compared to that in 2004-2009. This finding was similar to a that of a study which was done in Northern India, where out of 115 MRSA, 73% strains had shown multi-resistance [24]. Among other reports, Shrestha et al., had reported 97% MORSA [23]. However, their sample size was less (65 out of 67 strains). In another study which was done in 2006, multi-drug resistance was observed among 63.6% MRSA isolates which were obtained from clinical samples and among 23% MRSA isolates which were obtained from carriers. These results indicated a slowly emerging resistance of MRSA strains to several non-beta lactam drugs in different parts of India [25].
In conclusion, our study showed the changing patterns of antimicrobial resistance of MRSA strains in our hospital, which were consistent with the findings of studies which were done in other parts of the country. MRSA had displayed an increase in resistance to most of the antibiotics except tetracycline, along with a substantial increase in multi-resistant MRSA strains over a period of seven years. Although all MRSA strains showed in vitro sensitivities to vancomycin and had MICs in sensitive range, isolates which were recovered during 2010 and 2011 showed marginal increases in MICs as compared to those of older isolates. Resistance to co-trimoxazole, ciprofloxacin and gentamicin was high in both recent and old MRSA isolates and therefore, these drugs are not suitable for empirical therapy of suspected Staphylococcal infections. We conclude that S. aureus is a pervasive pathogen in our hospital and in community settings with constantly changing trends in virulence, resistance and epidemiology and thus, monitoring of clinical and microbiological parameters is necessary, for modifying our existing infection control measures and treatment options accordingly.