Sclerosing stromal tumours (SSTs), which were defined by Chalvardjan and Scully [1] in 1973 for the first time, are rare, stromal and benign tumours of ovary. SSTs constitute 6% of the tumours that are derived from the stroma of ovary and more than 80% of such tumours are observed in young adult women in the 2nd and 3rd decades of life [2–5]. Sclerosing stromal tumours are usually hormonally inactive, but it has been reported that some cases are related to pregnancy, androgenic symptoms and endometrial carcinomas. The most frequent presenting complaint is menstrual irregularity and pelvic pain. Macroscopically, they are usually observed as solid and typically unilateral tumours [6–9].
The sharpest histological finding is the pseudo-lobular pattern that is formed by the cellular nodules that are separated from each other by hypocellular, oedematous and collagenous stroma [10]. The hemangiopericytomatous pattern–like dilated vascular structures are the characteristics of cellular areas, and sometimes, they can be associated with angiomatous lesions [11]. In microscopic examinations, the luteinized theca–like cells with vacuolized cytoplasm and fusiform fibroblast–like cells point out in hypercellular areas.
In this study, 7 SST cases who were aged between 18–25 years, who were diagnosed in our hospital, were examined morphologically, clinically and immunohistochemically (IHC) and were reviewed together with the literature data.
Methods
Seven cases who were aged between 17–25 years with a diagnosis of SST were selected from the files of our hospital between 2001 and 2011. The operational materials of all the cases were examined. The clinical and macroscopic data of the cases were obtained from our archival records and all the archival preparations which were stained with hematoxylin–eosine were reviewed. A block which represented the SST diagnosis best was selected from each case and an immunohistochemical method was performed. The primary antibodies that were used were those for oestrogen receptor (ER), progesterone receptor (PR), inhibin, calretinin, melanA, CD10, smooth muscle actin (SMA), desmine, vimentin, CD34, S–100, C–kit, cytokeratin (CK) and cytokeratin7 (CK7).
Immunoreactive cells were evaluated according to their staining densities and the percentage of positive cells (weak, 1+; moderate, 2+; strong, 3+). A positive control was used for each primary antibody.
Results
Clinical Findings
The ages of the patients varied between 18 and 25 years (mean age- 20 years). Clinically, menstrual irregularities were detected in 2 patients, abdominopelvic pain was detected in 2 patients, and pregnancies were detected in 3 patients. No virilisation was observed. Although SSTs are usually hormonally inactive, most of our cases had occurred together with pregnancies and menstrual irregularities. All the tumours were unilateral. Five tumours were located in the right ovary and 2 tumours were located in the left ovary.
CA125 tumour markers were within normal limits. All the cases were processed with frozen sections, 4 cases were underwent laparoscopic oopherectomies, and the other 3 patients underwent laparotomical adnexal mass excisions. Patients were followed for a period of 1 to 5 years (mean age–4 years) post–operatively. Clinical findings and surgical procedures have been shown in [Table/Fig-1].
Clinical findings and surgical procedures
| S. No. | Age | Clinic | CA–125 | Side | Tumour size (cm) | Gross appearance | Surgery |
|---|
| 1 | 21 | pregnancy | Normal | right | 8.5 | Solid | laparoscopy |
| 2 | 19 | pregnancy | Normal | left | 6 | Solid | laparoscopy |
| 3 | 19 | Menstruel irregularity | Normal | left | 6 | Solid | laparoscopy |
| 4 | 25 | Menstruel irregularity | Normal | right | 9 | Solid | laparotomy |
| 5 | 25 | Abdominal-discomfort | Normal | right | 10 | Solid | laparotomy |
| 6 | 19 | pregnancy | Normal | right | 6 | Solid–cystic | laparoscopy |
| 7 | 18 | Abdominal-discomfort | Normal | right | 12 | Solid–cystic | laparotomy |
Macroscopic Findings
The sizes of the tumours were between 6 cm and 12 cm (mean size–8.2 cm). All the tumours were observed within good margins. Five tumours were in solid structures on the incision surface, and 2 tumours were composed of solid and cystic areas. The diameters of these cystic areas varied between 0.1 and 8 cm, and there was clear liquid in the cysts. The solid components were white to yellowish–pink in nodular areas.
Microscopic Findings
All the tumours were surrounded by fibrous capsules and they were located within good margins. The irregular distribution of tumour cells resulted in cellular pseudolobules that were separated by hypocellular areas which had resulted from oedema and hyalinization. Two different types of cells were exhibited in the cellular areas. One type was fibroblast–like fusiform cells, and the other was luteinized the ca–like cells with a vacuolized cytoplasm. In some cases, the nuclei in these cells were eccentrically located in some places and they reminded of signet ring cells. No mitosis was detected. Nuclei had a vesicular appearance, and sometimes nuclei were evident.
Small–medium sized and thin–walled ectatic blood vessels were detected, especially in the cellular component. These vessels had a hemangiopericytomatous pattern and some were shaped like a boomerang. Necrosis was observed only in 2 cases and there was hyalinization and calcification in 2 cases.
Immunohistochemical Findings
A diffuse positivity was detected in all tumours with vimentin.
Both SMA and desmin stained strongly in fibroblast–like cells, but no staining was observed in the areas where the theca-like cells were dominant.
Inhibin [Table/Fig-2] and Calretinin [Table/Fig-3] were positive in theca-like vacuolized cells.
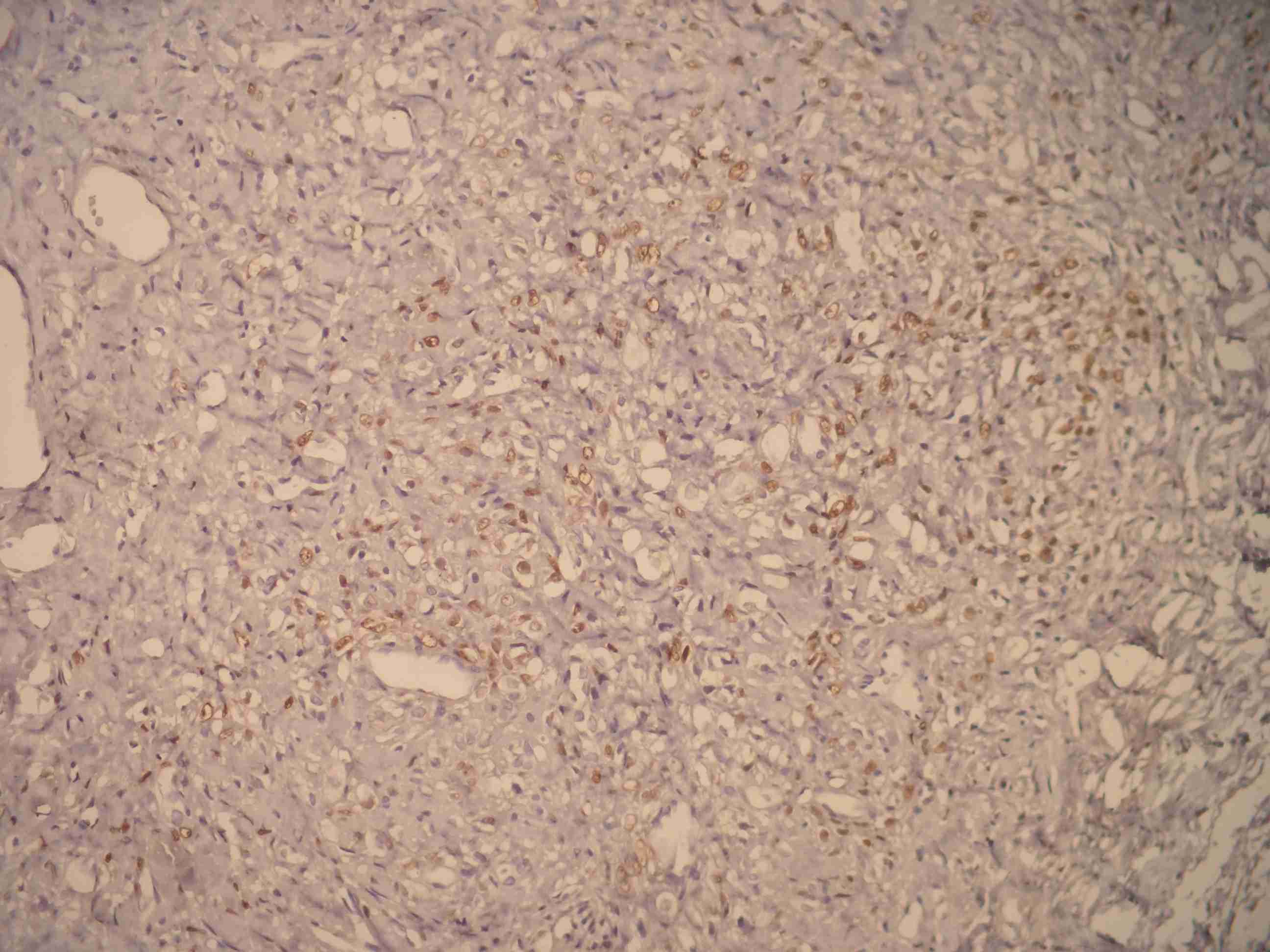
Calretinine positive cells
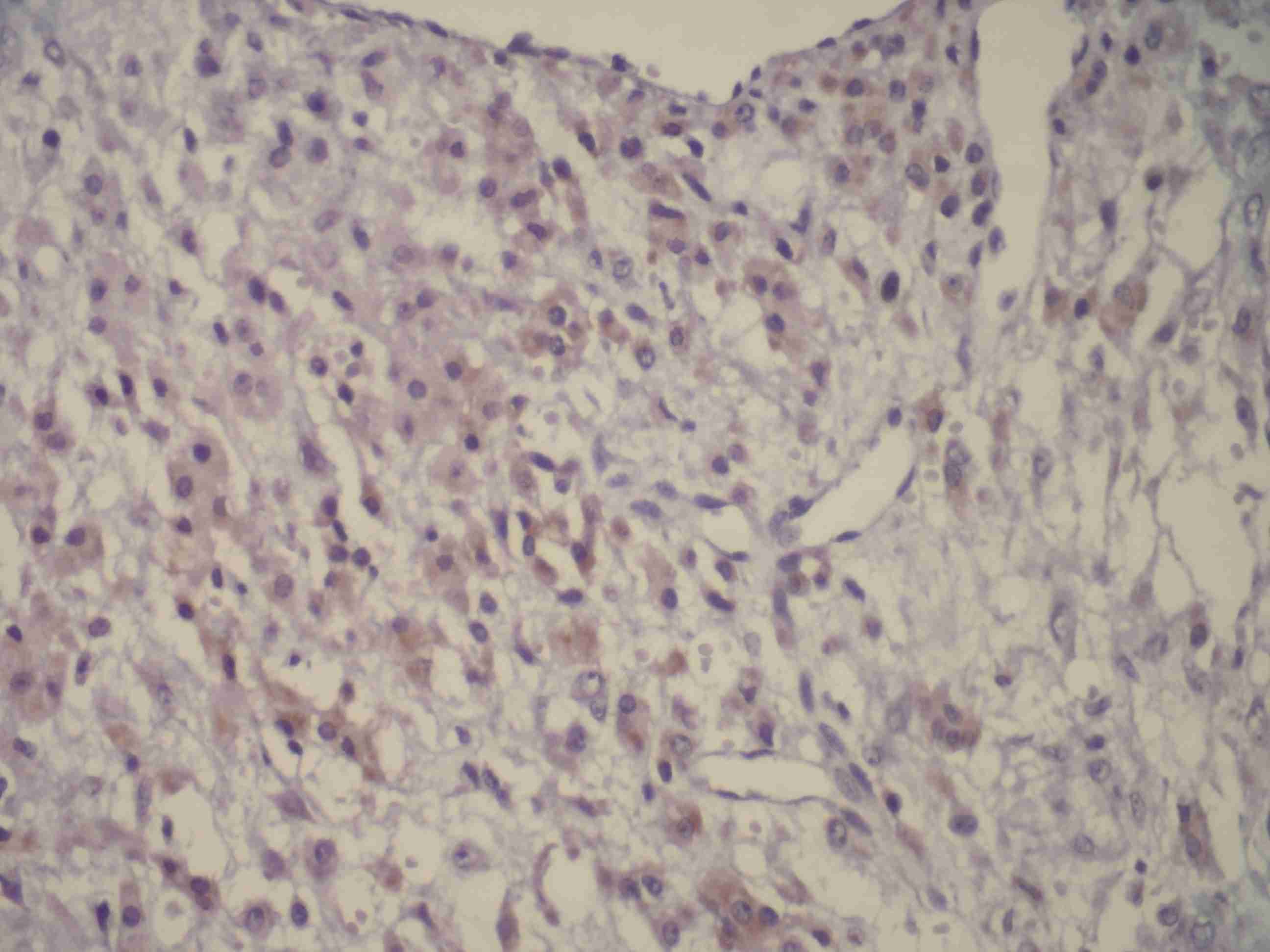
While PR was focally positive in all the cases , ER was not positive in any cases.
C–kit was weakly positive in 5 cases, melan-A [Table/Fig-4] was weakly positive in 4 cases and CD–10 was weakly positive in 3 cases in focal areas.
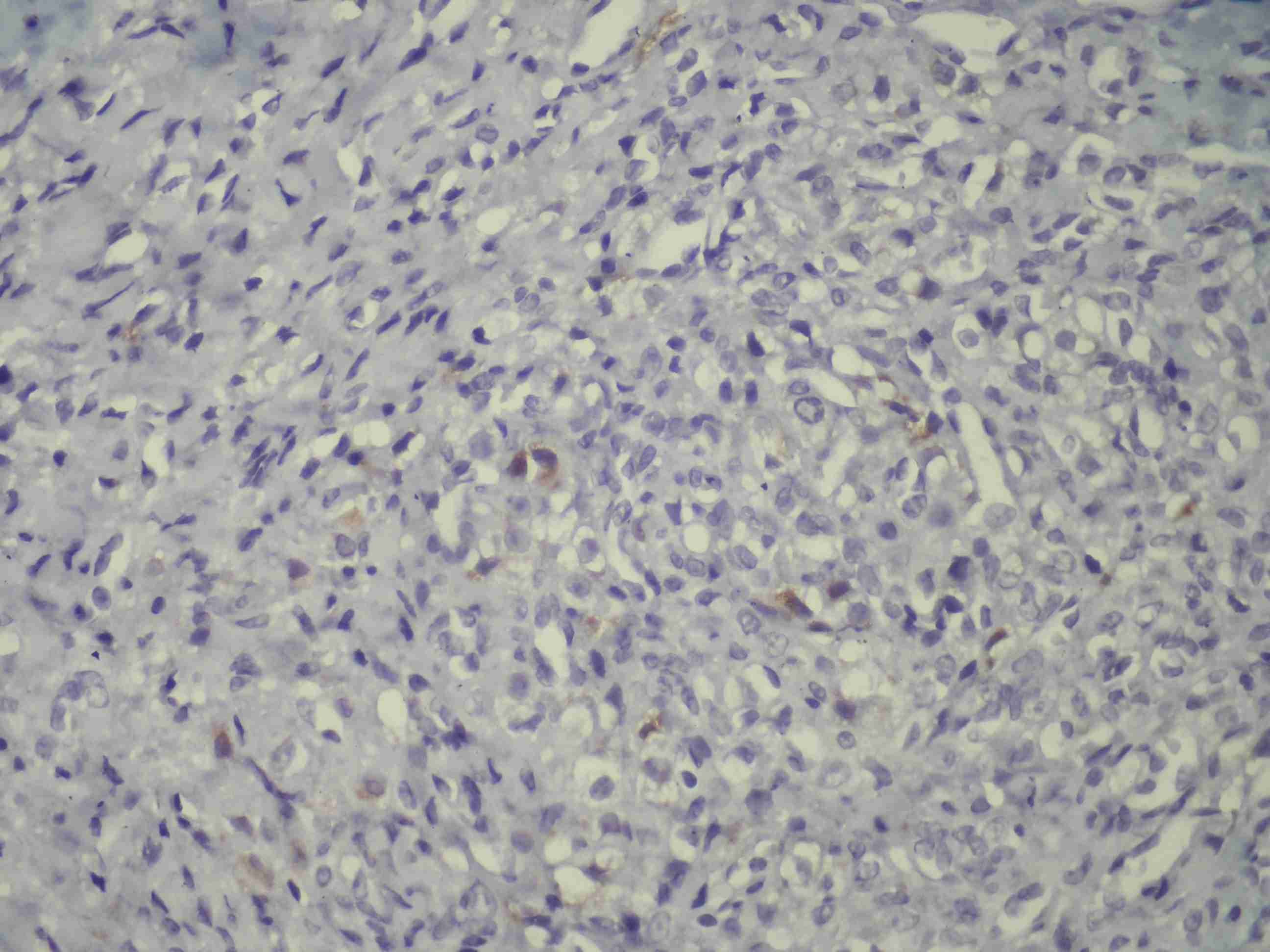
No staining was observed in our cases with CK and CK–7.
The immunohistochemical profile of our cases has been shown in [Table/Fig-5].
The immunohistochemical profile of our cases
| (P/I) | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| Vimentin | 90/3+ | 80/3+ | 70/3+ | 80/3+ | 70/3+ | 90/3+ | 80/3+ |
| SMA | 80/3+ | 70/3+ | 90/3+ | 90/3+ | 70/3+ | 70/3+ | 90/3+ |
| Desmin | 45/3+ | 60/3+ | 40/3+ | 50/3+ | 45/3+ | 55/3+ | 40/3+ |
| Inhibin | 30/3+ | 10/2+ | 10/2+ | 30/3+ | 40/3+ | 30/2+ | 30/3+ |
| Calretinin | 90/3+ | 40/1+ | 80/1+ | 30/3+ | 30/2+ | 5/3+ | 30/3+ |
| PR | 35/1+ | 40/2+ | 10/2+ | 40/3+ | 10/3+ | 30/3+ | 10/3+ |
| ER | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| C-kit | 10/2+ | 10/1+ | 5/2+ | 1/2+ | (-) | (-) | (-) |
| Melan-A | (-) | 10/2+ | (-) | 10/1+ | 10/2+ | 5/1(+) | (-) |
| CD-10 | 20/2+ | (-) | 20/2+ | 10/2+ | (-) | (-) | (-) |
| CK | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
| CK-7 | (-) | (-) | (-) | (-) | (-) | (-) | (-) |
P: percentage of positive cells/ I: intensity of staining
Discussion
Ovarian neoplasms are rare in adolescents and they are mainly tumours of germ cell origin. SSTs of the ovary are of sex–cord stromal origin, that occur in young women with a mean age of 28 years. This type was defined by Chalvaridjan and Scully (1973) for the first time and it is often observed in the 2nd and 3rd decades of life [1]. To the best of our knowledge, although this tumour has been described before in adolescents, only one paediatric case of a bilateral SST (in a 10 year old premenarchal girl) has been described in the clinical literature [12]. An overview on all case reports on SSTs which had been reported between 2003–2013, has been shown in [Table/Fig-6].
Overview of all case reports on SST between 2003–2013
| Article | Patient Age | Side | Clinic | CA–125 | Tumor size (cm) | Gross apperance | Immunohistochemical staining |
|---|
| (1) Fefferman NR et al., 2003 | 10 | L | Pain, vague, voiding | N | 13 x 10 | Solid, multilocule | |
| (2) Peng HH et al., 2003 | 24 | L | Irregulary menstruation | | | Solid | |
| (3) Kim JY et al., 2003 (3 cases) | 16 | L | Irregulary menstruation | N | 6x5 | Solid | SMA(+), Vimentin (+), PG(+) |
| 26 | L | Vaginal bleeding, menorrhagia | N | 6x5 | Solid, | SMA(+), Vimentin focally (+) |
| 39 | L | Irregulary menstruation | N | 5,5x4,5 | Cystic | |
| (4) Kuscu E et al., 2003 | 34 | R | Hirsutism, oligomenorrhea | N | | Solid | SMA(+), CK(-), Keratin(-), S100(-), desmin(-) |
| (5) Yerli H et al., 2003 | 34 | L | Amenorrhea hirsutism | N | 12,5x10 | Solid | Actin focally (+), CK(-), S100 (-), Desmin (-). |
| (6) Deval B et al., 2003 | 29 | R | | N | 4,5x4 | Solid, cystic, hemorrhagic | |
| (7) Calabrese M et al. | 30 | R | Pregnancy, pelvic pain, dysuria | N | 14 | Cyst | |
| (8) Bildirici K et al.,2004 | 14 | | | | | Solid, calsified | |
| (9) Gurbuz A et al., 2004 | 21 | | Pregnancy | | 6x7 | Solid | Vimentin(+), SMA (+), Desmin(+) |
| (10) Bouraouis et al., 2004 (3cases) | 15 26 56 | | | | | | |
| (11) Popovska S et al., 2005 | 26 | | | | | | |
| (12) Korczyinski J et al.,2005 | 30 | | Irregulary menstruation, pelvic pain | N | | | |
| (13) Terauchi F et al. | 18 | | | High | | | |
| (14) Chang W et al., 2006 | 11 | B | | N | Left 8,5x6; right 17x12 | Solid, cystic | SMA (+) |
| (15) Pai R et al., 2005 (4 cases) | Mean 22 | | Aymptomatic mass (2) Menorrhagia(1) Amenorrhea(1) | | | Solid-cystic (2) Solid (1) Cystic(1) | |
| (16) Akyildiz EU et al., 2004 (3 cases) | 23 24 28 | | | | | | |
| (17) Charfi Darghouth L. et al., 2007 | 15 | | | | | | |
| (18) Iravanloo G et al., 2008 | 26 | L | Pelvic pain Irregulary menses Abdominal distension | N | 23x8 | | |
| (19) Ergeneli MH et al., 2008 | 11 | | | | | | |
| (20) Chang YW et al., 2009 | 12 | B | Palpable mass | N | Right 13 Left 2 | Solid, calcification | |
| (21) Youm HS et al., 2008 | 71 | L | Abdominal distention dyspnea | N | 44x33 | Multicystic, solid, necrosis | Desmin(+), SMA(+),S100(-) |
| (22) Wada H et al., 2009 | 52 | L | Abdominal distention | N | 31x23 | Solid | |
| (23) He Y et al., 2010 | 4 | | Premature thelarche | | | | |
| (24) Amorim-Costa C et al., 2010 | | | Meigs’ syndrome | High | | | |
| (25) Park SM et al., 2011 | 11 | L | Hirsutism Deepening of the voice | | 9x7,5 | Solid | Vimentin(+), SMA(+), Inhibin(+), S100(-), CK(-) |
| (26) Banik T et al., 2012 (3 cases) | 19 | L | Menometrorrhagia, abdominal pain | | 5x4 | Solid | |
| 21 | R | Menometrorrhagia | | 12 | Solid Cystic | |
| 18 | | Abdominal pain | High | 8,5x5 | Solid | Inhibin focal(+), CK(-), Vimentin(-), SMA(-), Desmin(-), EMA(-) |
| (27) Chung CP, et al., 2012 | 59 | L | Recurrent vaginal bleeding | | 1,5 | Solid | |
| (28) Khanna M et al., 2012 | 32 | | Menstruel irregularities Pelvic pain | High | 16x13 | Solid, small cystic spaces | SMA(+) |
| (29) Boussaid K et al., 2013 | 24 | L | McCune Albright syndrome, hirsutisme, acne, amenorrhea | | | Solid | Inhibin(-), vimentin(-), CK(-), WT1(+) |
Sclerosing stromal tumours are rarely seen together with pregnancies; only 8 pregnant patients have been reported in literature so far. According to the literature, our 3 pregnant patients are the reported 9th, 10th and 11th patients.
Clinically, there was menstrual irregularity in 2 cases and abdominopelvic pain in 2 cases, and 3 cases were pregnant. These findings made us think that these tumours were hormonally active. In the literature, premenarchal, pregnancy women, virilization, cliteromegaly, amenorrhe were presented.
Macroscopically and microscopically, characteristic histopathological findings of the SSTs of ovary were observed in all the cases.
The hormonal activities of these tumours have been presented before. Lam and Geittman [13] proved that these tumours synthesized dehydroepiandrosterone and that when steroidogenesis occurred, it was usually oestrogenic, which caused irregular menses, amenorrhoea, and infertility. A precocious puberty in childhood has also been reported. A virilizing SST of the ovary in a young woman with Mc Cune Albright Syndrome was reported in 2012 by Bousssaid K et al., [14] and a woman with irregular menstruation was reported by Khanna M et al., [15]. To date, eight cases of virilizing SSTs have been described in the literature and two tumours were diagnosed after clomiphene treatment for infertility in patients whose ovaries had been laparoscopically normal before [16]. Lifschitz–Mercer et al., proved that PR stained positively in SST cells [17]. Kostopoulou E et al., [18] defined that a positivity for ER beta was observed in a significantly larger number of cells than that for ER alpha [18]. In our study, while ER was negative in all the cases, PR was confirmed to be positive. Although the plasma levels of our cases were not measured, PR positivity on IHC made us think that these tumours could be hormonally active.
Saitoh et al., [19], Shaw et al., and Kawauchi et al., [6] have defined the smooth muscle cells in SSTs before. Shaw et al., [20] emphasized that SSTs stemmed from the perifollicular myoid stromal cells that were normally present in the theca externa. The IHC and ultrastructural results of the study of Santini et al., [21] proved that the smooth muscle differentiation was a component of the specialised gonadal stromal tissue. In our study, SMA and desmin were found to be as positive in fibroblast–like cells on IHC in all the cases. These findings supported the findings which were seen in the literature.
The IHC markers in the sex–cord stromal tumours, such as inhibin, calretinin, melan–A, WT–1, CD99 and mullerian inhibiting substance, were studied for making the differential diagnosis of SST [22–23]. According to the results of these studies, a correlation was observed between the calretinin and inhibin expressions and the luteinization level of tumour [24].
In the sex–cord stromal tumours of ovary, calretinin is a more sensitive, but a less specific marker than inhibin [22]. Mc Cluggage and Maxwell [23] observed calretinin positivity in only one tumour. Kommoss et al., [25] observed inhibin positivity in 4 of 11 cases. In our study, calretinin and inhibin were detected as positive in luteinized, theca–like cells with vacuolized cytoplasm in all the cases.
Jungbluth et al., [26] detected melan-A expression in normal gonadal sex–cord and stromal cells and in the cells that produced steroid hormone in adrenal cortex. These researchers detected melan-A positivity in 4 Leydig-cell tumours and 3 Sertoli–Leydig–cell tumours [27]. So, they emphasized that melan-A immunoreactivity could be used as a determinant in the differential diagnosis of the tumours that produced steroid hormones. Stewart et al., [28] proved that melan-A was a moderately sensitive and a specific marker that showed the sex-cord stromal differentiation. In the study of Stewart et al., [28], they detected strong positivities in the hilus cells in normal ovaries, and focal positivities in the tubular cells of rete ovaries in 2 cases. So, they thought that melan-A reactivity could probably be existent in normal and hyperplastic cortical stromal cells In our study, melan-A ranged perivascularly, similar to the inhibin staining, in 4 cases.
CD10 is a marker that is used in detection of endometrial stromal tumours. Oliva et al., [29] reviewed the CD10 expression in full stromal and sex-cord stromal tumours of ovary in 101 cases, and they observed that the frequency and density of CD10 staining in these tumours were much lower than those of the endometrial stromal tumours. They pointed out that among these tumours of ovary, steroid-cell tumours more often showed CD10 positivity as compared to the others. In 3 of our cases, CD10 was observed to be positive in the luteinized cells with vacuolized cytoplasm.
While no staining was observed in any case with CK, CK7 and S–100, it should be remembered that a CK negativity can be checked for distinguishing the signet ring–like cells in the histopathology of SSTs which are diagnosed as per Krukenberg diagnosis.
The significance of these tumours is that it is necessary to distinguish the histopathology in frozen sections, in order to protect the other adnexa, because of the characteristics which are observed at early ages.
Our findings support the conclusion that SSTs are benign tumours that stem from stroma and that they are hormonally active tumours as per clinical and IHC results, although no hormonal effect that could be supported with laboratory tests was observed.