Bronchial asthma is characterized by chronic airway inflammation and increased airway hyperresponsiveness leading to symptoms of wheeze, cough and dyspnoea. Prevalence of asthma is increasing steadily over the late part of the last century in countries with a western life style and is also increasing in developing countries [1–3]. It is estimated that 300 million people worldwide suffer from asthma and this figure is projected to rise to 400 million by year 2025 [4]. Asthma accounts for approximately 500,000 hospitalizations each year [5], with around 250,000 deaths annually attributed to the disease [4]. In India, asthma imposes a substantial burden; though there is a paucity of appropriate epidemiological data to determine prevalence for asthma in India, a multicenter study by the Asthma Epidemiology Study Group of the Indian Council of Medical Research found the prevalence of bronchial asthma in Indian adults to be 2.38% [6].
Inhaled corticosteroids are the most effective drugs for the treatment of asthma and they represent first-line therapy for all patients with persistent disease, irrespective of disease severity [7]. Studies have demonstrated their efficacy in reducing symptom, frequency and severity of asthma exacerbations and asthma mortality. The major advantage of inhaled therapy is that drugs are delivered directly into the airways producing higher local concentrations with significantly less risk of systemic side effects.
Inhaled corticosteroids are marketed with different delivery devices, which have different lung deposition properties, in-vivo dosage accuracy and dose variability [8]. The commonly used devices in India are dry powder inhaler(DPI), metered dose inhaler(MDI), metered dose inhaler with spacer (MDI-S),and nebulizers. With inhaled corticosteroids being the mainstay of anti-inflammatory treatment in asthma, it is necessary to determine the comparative efficacy of different corticosteroids delivered through different inhaler devices. The present study was undertaken to assess the relative efficacy of fluticasone propionate administered through different delivery devices to adult patients of chronic stable bronchial asthma as measured by pulmonary function test parameters.
Material and Methods
This prospective one month study was conducted from March, 2013 to April, 2013 among clinically diagnosed patients of chronic stable bronchial asthma from out patient department of Pulmonology, Rajiv Gandhi Institute of Medical Sciences, Kadapa, Andhra Pradesh, India. Individuals of either sex aged between 18-65 years, who where residents of the local area and had a history of bronchial asthma for at least 6 months comprised the study unit. Prior approval for the study from the institutional ethical committee was obtained and a written informed consent from all patients was taken. The procedures followed in this study were in accordance with the ethical standards of the institutional ethical committee on human experimentation and with the Helsinki Declaration of 1975 that was revised in 2000. Sample size was calculated to be thirty six on the basis of prior observations reported in a previous study [9], but assuming a loss to follow up cases of up to 25% , the initial recruitment was calculated to be fourty eight.
Inclusion criteria
The subjects fulfilling the following criteria were considered to be suffering from chronic stable bronchial asthma as defined by American Thoracic Society 1987 [10].
History suggestive of bronchial asthma.
No acute exacerbation (episodes of progressive increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms) within the past one month.
No history of receiving any corticosteroid therapy for past one month.
Baseline forced expiratory volume in one second (FEV1) less than 80% of predicted value.
Increase in FEV1 equal or more than to 12% and peak expiratory flow rate (PEFR) equal or more than to 20% of baseline value 15 minutes after bronchodilator therapy.
Exclusion criteria
Patients with past history of hypersensitivity to fluticasone propionate.
History of treatment of asthma within four weeks prior to study.
Pregnant and lactating females.
Subjects with hepatic, cardiac, renal and respiratory disorders.
Those with an upper respiratory tract or acute sinus infection within four weeks prior to enrollment.
Individuals with a smoking history of >10 pack-years.
Patients on immunotherapy who required a change in dosage regimen within 12 weeks prior to enrollment were also excluded.
All study subjects underwent pulmonary function tests before and one hour after drug administration. Inhaled salbutomol (Asthalin Rotacaps from Cipla) 200 μg was administered on first visit (day-1) to assess bronchodilator reversibility and to fulfill the criteria of bronchial asthma. A single dose of fluticasone propionate 250 μg was administered by dry powder inhaler (DPI – Flohale Rotacaps from Cipla) on the second visit (day-8), by metered dose inhaler (MDI - Flohale Inhaler from Cipla) on the third visit (day 15) and by metered dose inhaler with spacer (MDI-S, Zerostat V Spacer by Cipla) on the fourth visit (day 22). Finally on the fifth visit (day 29) a single dose of fluticasone propionate 1mg (Flohale respule from Cipla) by nebulizer (ReadyMist from Meher Health Care Corp.) was given. After a standardized initial evaluation, which included complete history taking, clinical examination, investigations, asthma symptom score and spirometry, patients were requested to follow up every week for 4 weeks.
The severity of Asthma was assessed by asthma symptom score in which the severity of five most important asthma symptoms (shortness of breath, chest tightness, wheeze, cough, mucus production) were scored on a scale of 0--3 depending on severity to assess the efficacy of the candidate drugs. The symptom severity was defined as follows: 0 = No symptoms; 1 =Mild (symptoms are present occasionally and patients can continue with daily activities); 2 = Moderate (symptoms are present most of the time but patients can perform daily activities); 3 = Severe/Incapacitating (symptoms are severe and affect daily activities or patient cannot do things that they normally can) [11].
Spirometry was done at the beginning of study. Before spirometry it was ascertained patient had not taken inhaled β2 agonist (salbutamol) for at least 6 hours and theophylline therapy for at least 24 hours. Spirometry was performed by a Maestros spirometer where flow measurements were done by using Terbium followed by computerized analysis. At least three spirometry maneuvers were done and highest FEV1 was noted. Patients who had FEV1, less than 80% of predicted value were administered inhaled salbutamol 200 μg by nebulizer. Fifteen minutes after salbutomol administration spirometry was repeated and those patients who had an increase of at least 12% absolute FEV1 and at least 20% PEFR were labeled as suffering from bronchial asthma and enrolled in the study. Pre and post medication pulmonary function test (PFT) reports were collected. Thus in all, patients had to visit the department for 5 times including nomination, registration and 4 follow up visits.
Statistical Analysis
Data entry and statistical analysis was done using statistically package of social science (SPSS) software (version 17.0). Paired t -test, ANOVA and post hoc turkey’s test were used. p values less than 0.05 were considered significant and less than 0.001 highly significant.
Results
A total of 48 patients were enrolled in the study out of which, two patients were lost to follow up after second visit, another two patients were lost after third visit and five patients were lost after fourth visit. None of the patients experienced an acute exacerbation of asthma during the study period. Thus finally nine patients were excluded due to loss to follow up and the data of the remaining 39 subjects (25 males and 14 females) was analyzed [Table/Fig-1].
Patients flow through various stages of study
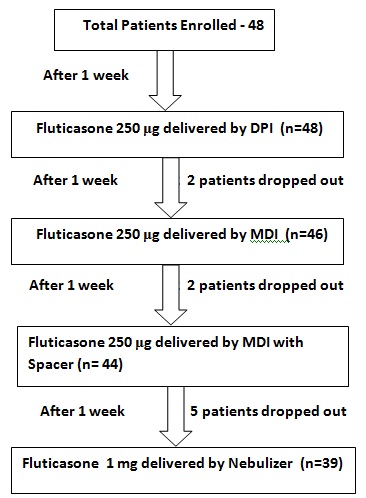
Twenty three (58.9%) individuals were aged between 18-40 years, 14 (35.9%) individuals were aged between 41-60 years and 2 (5.1%) individuals were aged between 61-65 years. The mean age of the patients was found to be 39.3 years.
Mean asthma scores calculated varied between 5.71 to 5.83 on various days of visits. There was no significant difference in patient’s asthma symptom score per week at day 1, 8, 15, 22 and 29 (p = 0.99). Since there was no significant change in pulmonary function test parameters (before giving fluticasone) at week-2, week-3, week-4, week-5, which shows that the patients were suffering from chronic stable bronchial asthma and there was no significant modification in the disease process during the course of the study. No significant change in the asthma symptom scores also shows that there was no acute exacerbation and the patients were stable [Table/Fig-2].
Asthma Symptom Score of the Patients at different visits
| Visit | Asthma Symptom Score (Mean ± SD) |
|---|
| Day 1 | 5.79 ± 1.02 |
| Day 8 | 5.74 ± 1.07 |
| Day 15 | 5.83 ± 0.95 |
| Day 22 | 5.77 ± 0.83 |
| Day 29 | 5.71 ± 0.98 |
| ANOVA f value p value | 0.09 |
| 0.99* |
S.D: standard deviation; * p value not statistically significant (> 0.05)
Pretreatment values of peak expiratory flow rate varied between 32- 48%, 33- 49 %, 31- 50 % and 33- 51 % before giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5) respectively. There was no significant difference in PEFR values at week 2, 3, 4 and 5, before giving the drug by different devices (p>0.05) [Table/Fig-3].
Peak Expiratory Flow Rate (PEFR) (% Predicted) before and after giving Fluticasone by different devices
| Delivery Device | PEFR |
|---|
| Pre-treatment Value (Mean± S.D) | Post-treatment Value (Mean± S.D) |
|---|
| DPI | 40.11 ± 3.93 | 44.52 ± 4.28 |
| MDI | 40.98 ± 4.09 | 44.89 ± 4.31 |
| MDI-S | 41.14 ± 4.29 | 45.33 ± 4.06 |
| Nebulizer | 40.73 ± 4.08 | 45.40 ± 3.96 |
| ANOVA | f value | 0.48 | 0.38 |
| p value | 0.70* | 0.77* |
PEFR: peak expiratory flow rate; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
Pretreatment values of forced expiratory volume in 1 second varied between 62 - 75%, 61 -76%, 63 -76% and 58 - 77% before giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5) respectively. There was no significant difference in FEV1 values at week 2, 3, 4 and 5, before giving the drug by different devices (p>0.05) [Table/Fig-4].
Forced Expiratory Volume in 1 Second (FEV1) (% Predicted) before and after giving Fluticasone by different devices
| Delivery Device | FEV1 |
|---|
| Pre-treatment Value (Mean± S.D) | Post-treatment Value (Mean± S.D) |
|---|
| DPI | 68.25 ± 3.18 | 72.39 ± 3.57 |
| MDI | 67.59 ± 4.02 | 72.79 ± 4.22 |
| MDI-S | 68.17 ± 3.98 | 73.41 ± 4.50 |
| Nebulizer | 68.31 ± 4.63 | 73.86 ± 4.81 |
| ANOVA | f value | 0.27 | 0.89 |
| p value | 0.85* | 0.44* |
FEV1 : forced expiratory volume in 1 second; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
Pretreatment values of forced vital capacity (FVC) varied between 83 - 99%, 82 – 100%, 84 - 102% and 81 - 101% before giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5) respectively. There was no significant difference in FVC values at week 2, 3, 4 and 5 before giving the drug by different devices (p>0.05) [Table/Fig-5].
Forced Vital Capacity (FVC) ( % Predicted) before and after giving Fluticasone by different devices
| Delivery Device | FVC |
|---|
| Pre-treatment Value (Mean± S.D) | Post-treatment Value (Mean± S.D) |
|---|
| DPI | 91.36 ± 4.41 | 96.15 ± 4.57 |
| MDI | 90.44 ± 4.68 | 95.30 ± 5.05 |
| MDI-S | 91.18 ± 5.19 | 96.77 ± 5.21 |
| Nebulizer | 91.27 ± 5.35 | 96.82 ± 5.67 |
| ANOVA | f value | 0.29 | 0.74 |
| p value | 0.84* | 0.53* |
FVC: forced vital capacity; PEFR: peak expiratory flow rate; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
Pretreatment values of FEV1 /FVC varied between 0.67 - 0.81%, 0.68 - 0.82%, 0.66 - 0.84% and 0.65 - 0.82% before giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5) respectively. There was no significant difference in FEV1 /FVC values at week 2, 3, 4 and 5, before giving the drug by different devices (p>0.05) [Table/Fig-6].
FEV1 / FVC (% Predicted ) before and after giving Fluticasone by different devices
| Delivery Device | FEV1/FVC |
|---|
| Pre-treatment Value (Mean± S.D) | Post-treatment Value (Mean± S.D) |
|---|
| DPI | 0.74 ± 0.05 | 0.75 ± 0.06 |
| MDI | 0.74 ± 0.06 | 0.76 ± 0.06 |
| MDI-S | 0.75 ± 0.06 | 0.76 ± 0.07 |
| Nebulizer | 0.74 ± 0.05 | 0.76 ± 0.06 |
| ANOVA | f value | 0.32 | 0.25 |
| p value | 0.81* | 0.86* |
FEV1 / FEC : ratio of forced expiratory volume in 1 second to forced vital capacity ; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
One hour after giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5), there was highly significant increase in PEFR (P<0.001). The post treatment value of PEFR was highest after giving fluticasone by nebulizer (40 - 52%) followed by dry powder inhaler (36 - 50%), metered dose inhaler with spacer (33 - 50 %) and metered dose inhaler (32 - 50 %). However there was no significant difference in the PEFR after giving fluticasone by any of the devices (p=0.77) [Table/Fig-3].
One hour after giving fluticasone by the different devices at week 2, 3, 4 and 5, there was highly significant increase in FEV1 (p<0.001). The post treatment values of FEV1 ranged between 68 -80%, 63 - 83%, 69 -81% and 66 - 83% by dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer respectively, the difference being statistically insignificant (p=0.44) [Table/Fig-4].
One hour after giving fluticasone by different devices at week 2, 3, 4 and 5, there was highly significant increase in FVC (p<0.001). The post treatment values The percentage change in FVC ranged between 83 - 102%, 85 - 104%, 86 -106% and 84 - 107% by dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer, the difference being statistically insignificant (p=0.53) [Table/Fig-5].
One hour after giving fluticasone by different devices at week 2, 3, 4 and 5, there was highly significant increase in FEV1/FVC (p<0.001). The percentage change in FEV1/FVC ranged between 0.64 - 0.82%, 0.69 - 0.83, 0.67 - 0.83% and 0.65 - 0.84% by dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer respectively, the difference being statistically insignificant (p=0.86) [Table/Fig-6].
The pulmonary function parameters showed a highly significant increase one hour after giving fluticasone by any of the devices evaluated. There was no significant difference in post–treatment values of peak expiratory flow rate (p=0.77), forced expiratory volume in one second (p=0.44), forced vital capacity (p=0.53) and forced expiratory volume in one second and forced vital capacity ratio (p=0.86) after giving fluticasone by dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer respectively at day 8, 15, 22 and 29. This shows a similar efficacy of fluticasone delivered via the different devices studied.
Discussion
To the best of our knowledge there is no Indian study comparing clinical efficacy of fluticasone delivered through various devices in patients of chronic stable bronchial asthma. Our study for the first time compared the effect of fluticasone delivered via dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer on lung functions and revealed that these devices have a similar effect on the lung function in patients of chronic stable bronchial asthma. Higher incidence of chronic stable bronchial asthma was found among those aged between 18 to 40 years in our study. This is in conformity with the results of previous surveys which show that bronchial asthma occurs in all ages with one half cases occurring before age of 10 years and another third before age 40 years. Out of 39 patients enrolled the majority (64.1%) were males in the current study. Previous studies however have shown that in adulthood prevalence of asthma is greater in women than men, reason for which is not clear [12]. The reason for higher enrollment of males in this study is partly due to relative reluctance of females in attending the hospital in this part of the world and partly due to exclusion of pregnant and lactating women from the study.
One hour after giving fluticasone by dry powder inhaler (week-2), metered dose inhaler (week-3), metered dose inhaler with spacer (week-4) and nebulizer (week-5) there was highly significant increase in PEFR in our study. The percentage change in mean peak expiratory flow rate was highest with nebulizer followed by dry power inhaler, metered dose inhaler with spacer and was least with metered dose inhaler [Table/Fig-7]. Several studies have demonstrated an increase in peak expiratory flow rate after giving fluticasone by various delivery devices over a period of 1 to 12 weeks [13–16].
Mean percentage change of spirometry parameters before and 1 hour after giving fluticasone propionate by various devices PEFR: peak expiratory flow rate; FEV1 : forced expiratory volume in 1 second; FVC: forced vital capacity; FEV1 / FEC : ratio of forced expiratory volume in 1 second to forced vital capacity
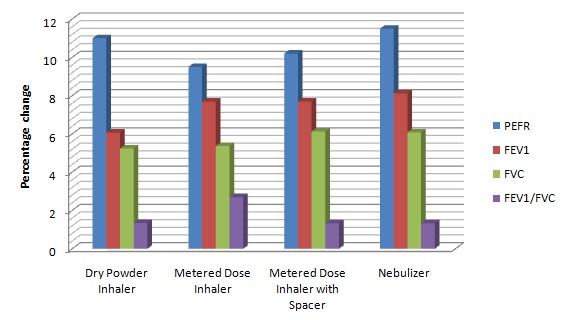
No significant difference in the PEFR was found after giving fluticasone by any of the different devices used in our study which is in agreement with the study done by Bateman et al., [17] in which there is no significant difference found with salmeterol/fluticasone propionate in combination (50/100 μg twice daily) when administered via a chlorofluorocarbon-free metered dose inhaler or dry powder inhaler to patients with mild-to-moderate asthma.
One hour after giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5), there was a highly significant increase in FEV1 in our study. Although the percentage change in forced vital capacity was highest with nebulizer, followed by metered dose inhaler and metered dose inhaler with spacer and least with dry powder inhaler [Table/Fig-7], there was no significant difference found in the FEV1 after giving fluticasone by any of the devices used in our study which is in line with study done by Koser et al., [18] in which they compared fluticasone propionate by MDI and DPI and found that there was no statistically significant difference in FEV1 with both the devices.
One hour after giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5), forced vital capacity also increased significantly. The percentage change in forced vital capacity was highest with MDI-S, followed by nebulizer and MDI and least with dry powder inhaler [Table/Fig-7] but there was no significant difference in the FVC after giving fluticasone by any of the devices. Nair et al., [19] compared inhaled fluticasone delivered via metered dose inhaler and three antistatic spacers and found no statistically significant differences in FVC.
One hour after giving fluticasone by DPI (week-2), MDI (week-3), MDI-S (week-4) and nebulizer (week-5), there was highly significant increase in forced expiratory volume in one second and forced vital capacity ratio (FEV1/FVC). The percentage change in FEV1 /FVC was highest with MDI, followed by DPI and was least with both MDI-S and nebulizer [Table/Fig-7]. There was no significant difference found in the FEV1/FVC after giving fluticasone by any of the devices. Previous studies on inhaled fluticasone by different devices in patients of chronic stable bronchial asthma have not reported the effect on FEV1/FVC. The present study found no significant differences on spirometric variables after giving fluticasone via nebulizer, metered dose inhaler, metered dose inhaler with spacer and dry powder inhaler.
The results obtained in our study in relation to the efficacy fluticasone propionate with regard to various PFT parameters are in accordance with the results of the systematic review of literature done by Brockleband et al., [20] in which there was no difference in clinical effectiveness of fluticasone propionate between nebulisers and alternative inhaler devices compared to standard MDI with or without a spacer device.
Conclusion
Fluticasone propionate delivered by different devices (dry powder inhaler, metered dose inhaler, metered dose inhaler with spacer and nebulizer) have similar effect on lung function in patients of chronic stable bronchial asthma and may be used interchangeably depending on availability, cost and compliance of the patients.
S.D: standard deviation; * p value not statistically significant (> 0.05)
PEFR: peak expiratory flow rate; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
FEV1 : forced expiratory volume in 1 second; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
FVC: forced vital capacity; PEFR: peak expiratory flow rate; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)
FEV1 / FEC : ratio of forced expiratory volume in 1 second to forced vital capacity ; DPI: dry powder inhaler; MDI; metered dose inhaler; MDI-S: metered dose inhaler with spacer; S.D: standard deviation; * p value not statistically significant (> 0.05)