Bromocriptine, a Dopamine (d2) Receptor Agonist, Used Alone and in Combination with Glipizide in Sub–Therapeutic Doses to Ameliorate Hyperglycaemia
Harish Kumar V.S.1, Vinutha M.B.2, Pradeep A.N.3, Sathisha Aithal4, Sindhura Reddy Baleed5, Umakant N. Patil6
1 Assistant Professor, Department of Pharmacology, S S Institute of Medical Sciences & Research CentreDavanagere, Karnataka, India.
2 Phase III MBBS Student, Department of Pharmacology, S S Institute of Medical Sciences & Research CentreDavanagere, Karnataka, India.
3 Assistant Professor, Department of Pharmacology, S S Institute of Medical Sciences & Research CentreDavanagere, Karnataka, India.
4 Associate Professor, Department of Pharmacology, S S Institute of Medical Sciences & Research CentreDavanagere, Karnataka, India.
5 Phase III MBBS Student, Department of Pharmacology, S S Institute of Medical Sciences & Research CentreDavanagere, Karnataka, India.
6 Professor & HOD, Department of Pharmacology, S S Institute of Medical Sciences & Research CentreDavanagere, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Mr. Harish Kumar V S, Assistant Professor, Department of Pharmacology, SSIMS & RC, NH-4, Davanagere, Karnataka, India.
E-mail: harishkvspharma@gmail.com
Introduction: Bromocriptine, an ergot derivative, is an agonist at the dopamine 2 receptor and a sympatholytic. It is a well established drug in Parkinsonism, hyperprolactinaemia and acromegaly and it has various other clinical indications like induction of ovulation in female infertility. Bromocriptine has been evaluated in alloxan induced diabetic rats for its anti-hyperglycaemic effect with and without simultaneous use of glipizide.
Methods: Diabetes was induced in albino rats by giving a single subcutaneous injection of alloxan in a dose of 150 mg/kg body weight. After 72 hours of giving alloxan injection, depending upon their blood glucose levels (350mg/dl and above), the rats were included into the study and they were divided into four groups, each comprising of 6 rats (n=24): Group 1 which was taken as control was given distilled water. Group 2 was treated with glipizide, a standard drug. Group 3 was treated with the test drug, bromocriptine and Group 4 was treated with sub therapeutic doses of test and standard drugs. The drugs were given to the diabetic rats once daily by oral route for 30 consecutive days, in order to assess their effects in terms of reduction in blood glucose levels. Blood glucose was estimated on 0th, 10th, 20th, and 30th days of the study at fixed time intervals.
Results and Conclusion: Bromocriptine, which was used alone, lowered the blood glucose levels appreciably; whereas the concomitant administration of bromocriptine and glipizide in sub therapeutic doses produced a much more appreciable reduction. The results which were obtained in the group which received simultaneous administration of test and standard drugs in sub therapeutic doses were comparable to those of the group which received reference drug, glipizide. Hence, it can be concluded that bromocriptine may serve as a valuable adjunct to available anti-diabetic medication.
Bromocriptine, Anti-hyperglycaemic, Glipizide, Alloxan
Introduction
Diabetes is an “iceberg” disease. The increasing worldwide incidence of Diabetes Mellitus (DM) in adults is a global public health burden. Diabetes mellitus is a group of heterogenous disorders in which carbohydrate metabolism is reduced, while those of proteins and lipids are increased. Hyperglycaemia is a common end point for all types of DM and it is an important parameter which can be assessed to evaluate the effectiveness of anti-diabetic drugs. The goals of therapy for diabetes are to alleviate the symptoms which are related to hyperglycaemia and to prevent or reduce the acute and chronic complications of diabetes. Because of its multi-factorial pathogenesis, restoration of normoglycaemia is difficult to achieve and it requires multiple anti-diabetic medications in combination, to produce an additive effect. Therefore, the development of anti-diabetic agents that have novel mechanisms of action and can be used in combination with currently approved medications for the treatment of type 2 Diabetes mellitus, is highly desirable.
The idea of using bromocriptine for the treatment of type-2 diabetes came while the metabolism of migratory birds was studied, in which they develop seasonal insulin resistance and dopamine plays a role in it [1]. This dopamine agonist is thought to potentially decrease insulin resistance and hepatic glucose production through an increase in dopaminergic neurotransmission, which can reset the hypothalamus and improve insulin sensitivity [2]. As dopaminergic activity is also related to the food intake and energy expenditure [3], it is thought that this dopamine receptor agonist plays a role in bringing down hyperglycaemia.
Hence, the present study was undertaken to explore the possible anti-hyperglycaemic action of bromocriptine, which may be an addition to its therapeutic utility. Our objectives were as follows:
To study the anti-hyperglycaemic activity of bromocriptine.
To compare its anti-hyperglycaemic activity with that of glipizide, which is an established anti-diabetic drug.
To study the anti-hyperglycaemic activity of sub therapeutic doses of bomocriptine and glipizide in combination.
Material and Methods
Animals: Albino rats which were 12 weeks old, which were of either sex, which weighed 150-200 grams, were bred locally in central animal house of S.S.I.M.S and R.C, Davanagere, Karnataka, India, and were used in the study. This study was approved by the institutional animal ethical committee of S.S.I.M.S and R.C., Davangere. The animals were housed in polypropylene cages and they were maintained under standard laboratory conditions at an ambient temperature of 23±2°C, with 12 hour light/12 hour dark cycles. Rats were fed with pellet chow diet and water ad libitum.
Drugs and Chemicals: Alloxan, dextrose, bromocriptine Mesylate, glipizide and Ether.
Instrument and other requirements: A glucometer (ACCU-CHEK ACTIVE) with strips, which was manufactured by- Roche Diagnostics GmbH, D-68298 Mannheim, Germany, Batch No- 91000247 and heparinized micro capillary tubes.
Experimental Procedure:Induction of Diabetes: Albino rats of either sex were injected with alloxan in a dose of 150 mg/kg body weight. Alloxan was freshly dissolved in sterile normal saline and it was administered subcutaneously [4, 5]. Animals were treated with 10% dextrose orally, to combat the early phase of hypoglycaemia [6]. After 72 hours of alloxan treatment, the rats which showed blood glucose levels of 350mg/dl and above were included in the study and they were divided into four groups of six animals each.
Group 1 (Control): treated with 0.5ml of distilled water p.o.
Group 2 (Standard): treated with glipizide 2.5mg/kg body wt p.o. [7].
Group 3 (Test): treated with bromocriptine 4.0 mg/kg body wt. p.o.[8].
Group 4 (Sub therapeutic): treated with the sub therapeutic doses of both test (bromocriptine in a dose of 2 mg/kg body wt) and standard (glipizide in a dose of 1.25mg/kg body wt) drugs p.o.
All the four groups were treated respectively, once a day, for a period of 30 days.
Sample collection and blood glucose estimation: Blood samples were collected from the retro-orbital plexuses of the rats after induction of mild anaesthesia with ether [9], on 0th, 10th, 20th and 30th days, at intervals of 0th min (before drug administration), 30th min, 1st hr, 2nd hr, 3rd hr, 6th hr, 12th hr and 24th hour after the drug administration on each of those particular days [10]. Blood sugar level was estimated by using a glucometer.
Statistical Analysis
The results were expressed in Mean ± SD and they were analyzed by repeated measures ANOVA test. The effects of bromocriptine alone and of its combination with glipizide were compared with the anti-diabetic activity of glipizide.
Results
As per [Table/Fig-1 and 2]:
Control Group- Effect of Distilled Water (0.5ml) on Blood Glucose Level (mg/dl) in Alloxanised Hyperglycemic Rats
| 0th minute | 30th minute | 1st hour | 2nd hour | 3rd hour | 6th hour | 12th hour | 24th hour | p* Value, sig |
|---|
| 0th Day | 376.8±15.5 | 377.0±21.8 | 379.5±18.2 | 379.8±21.3 | 379.7±27.7 | 384.5±19.1 | 382.5±18.9 | 383.5±14.8 | 0.84 NS |
| 10th Day | 383.0±19.6 | 382.5±18.4 | 383.8±17.3 | 379.8±21.3 | 365.7±48.0 | 384.5±19.1 | 382.5±18.9 | 383.5±14.8 | 0.54 NS |
| 20th Day | 385.8±19.0 | 384.7±21.5 | 385.5±8.8 | 384.7±18.9 | 382.7±16.7 | 380.5±14.5 | 382.8±19.1 | 386.5±18.6 | 0.97 NS |
| 30th Day | 388.2±14.7 | 387.2±15.4 | 391.2±10.8 | 392.2±18.7 | 388.7±18.6 | 391.3±18.6 | 396.7±20.3 | 399.2±17.2 | 0.41 NS |
Values are means ± SD, *Repeated measures ANOVA test, NS= Non significant
Blood glucose levels seen in control group at fixed time intervals on the days of blood glucose estimation
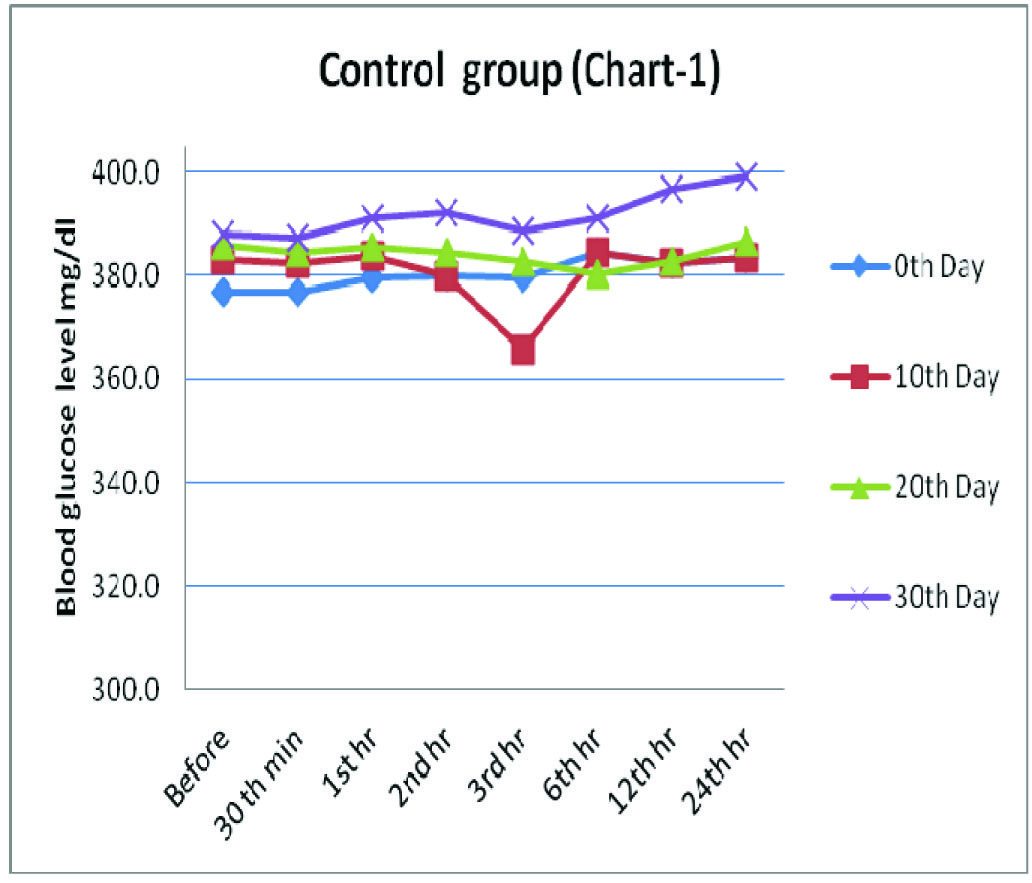
Group1 which was treated with distilled water served as negative control, wherein there was no marked variation in the blood sugar levels during the study period of 30 days, though slight alterations which lacked statistical significance were observed.
As per [Table/Fig-3 and 4]:
Standard Group- Effect of Glipizide (2.5 mg/kg body weight) on Blood Glucose Level (mg/dl) in Alloxanised Hyperglycemic Rats
| 0th minute | 30th minute | 1st hour | 2nd hour | 3rd hour | 6th hour | 12th hour | 24th hour | p * Value, sig |
|---|
| 0th Day | 387.3±11.3 | 381.7±9.9 | 375.5±7.6 | 363.7±6.9 | 360.5±11.7 | 352.0±9.5 | 347.3±9.6 | 342.7±11.3 | <0.001 HS |
| 10th Day | 337.7±3.3 | 335.0±3.4 | 330.8±5.2 | 321.7±7.8 | 317.2±6.6 | 311.7±6.0 | 310.2±7.2 | 304.8±6.2 | <0.001 HS |
| 20th Day | 273.8±8.3 | 271.7±7.8 | 267.2±8.3 | 261.5±7.4 | 255.3±8.5 | 249.5±8.7 | 242.8±7.1 | 235.2±10.3 | <0.001 HS |
| 30th Day | 205.5±8.4 | 203.3±8.1 | 191.3±12.0 | 183.2±11.2 | 177.3±7.4 | 171.0±6.8 | 161.5±8.3 | 154.3±7.0 | <0.001 HS |
Values are means ± SD, *Repeated measures ANOVA test, HS= Highly significant
Blood glucose levels seen in standard group at fixed time intervals on the days of blood glucose estimation
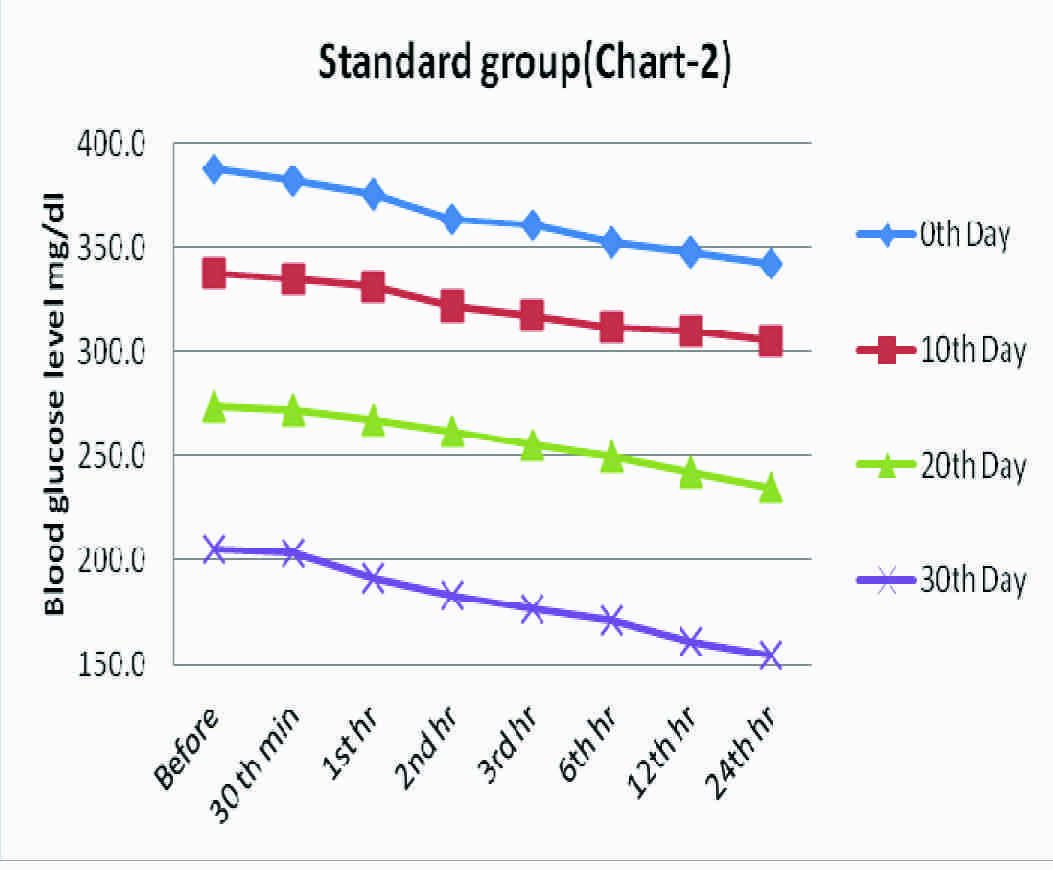
Group 2, a standard group, served as a positive control, which was treated with glipizide 2.5 mg/kg body wt, which showed a gradual reduction in the blood glucose levels in the span of 30 days of treatment. The blood glucose level which was 387.3 mg/dl before the start of experiment decreased up to 154.3 mg/dl at the end of 30th day, which implied a high statistical significance (p<0.001).
As per [Table/Fig-5 and 6]:
Test Group- Effect Of Bromocriptine (4 mg/kg body weight) On Blood Glucose Level (Mg/dl) In Alloxanised Hyperglycemic Rats
| 0th minute | 30th minute | 1st hour | 2nd hour | 3rd hour | 6th hour | 12th hr | 24th hr | p* Value, sig |
|---|
| 0th Day | 388.5±17.3 | 387.2±15.4 | 385.5±14.4 | 378.7±12.2 | 372.0±12.5 | 366.8±11.7 | 370.2±11.4 | 372.3±7.1 | <0.001 HS |
| 10th Day | 366.5±7.7 | 365.8±8.5 | 361.2±7.4 | 355.7±7.3 | 350.0±11.8 | 342.0±15.2 | 344.8±13.5 | 347.3±13.7 | <0.001 HS |
| 20th Day | 334.8±9.8 | 333.3±10.0 | 329.5±9.2 | 319.5±8.8 | 312.2±9.8 | 297.3±4.9 | 301.3±2.3 | 303.8±3.3 | <0.001 HS |
| 30th Day | 285.8±7.6 | 284.3±7.1 | 277.8±4.6 | 268.2±4.4 | 260.3±4.5 | 249.7±3.9 | 252.7±4.3 | 254.8±4.7 | <0.001 HS |
Values are means ± SD, *Repeated measures ANOVA test, HS= Highly significant
Blood glucose levels seen in test group at fixed time intervals on the days of blood glucose estimation
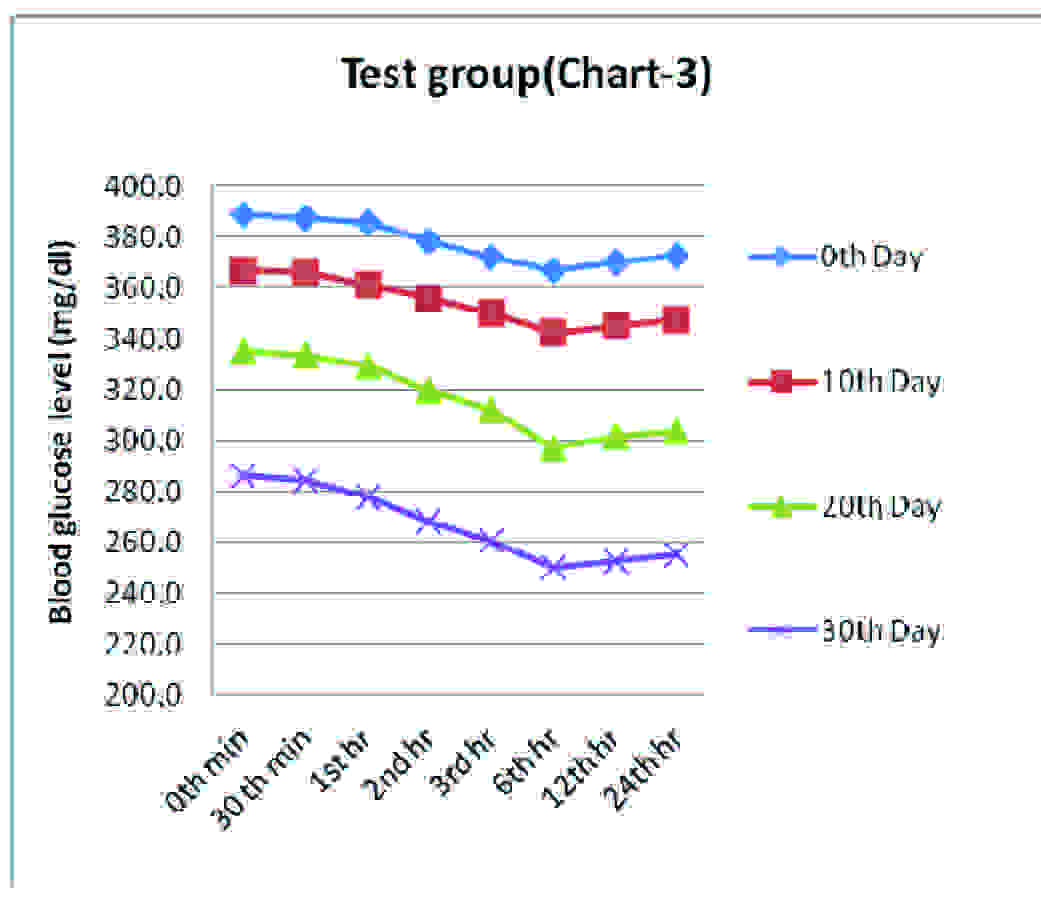
Group 3, bromocriptine 4.0 mg/kg body wt treated rats showed a gradual reduction in the blood sugar levels until 6th hour observations on the respective days of blood sugar estimation. There was a marked fall in the blood glucose level from 388.5 mg/dl on 0th day to 254.8 mg/dl on 30th day. The results, though they were statistically highly significant, (p<0.001) were not on par with the results which were obtained in case of Group 2.
As per [Table/Fig-7 and 8]:
Sub Therapeutic Group- Combination Effect Of Bromocriptine (2 mg/kg body weight) and Glipizide (1.25 mg/kg body weight) on Blood Glucose (mg/dl) In Alloxanised Hyperglycemic Rats
| 0th minute | 30th minute | 1st hour | 2nd hour | 3rd hour | 6th hour | 12th hour | 24th hour | p* Value, sig |
|---|
| 0th Day | 385.7±13.7 | 383.0±13.3 | 380.3±13.3 | 372.2±16.7 | 365.8±15.3 | 360.7±15.0 | 356.0±17.7 | 353.8±15.7 | <0.001 HS |
| 10th Day | 346.7±15.3 | 345.0±15.8 | 339.8±15.5 | 331.2±14.1 | 326.0±10.7 | 319.7±12.3 | 317.8±10.7 | 314.0±9.8 | <0.001 HS |
| 20th Day | 305.0±9.2 | 303.3±9.7 | 297.0±9.8 | 288.7±10.1 | 277.8±9.9 | 265.3±10.0 | 258.8±7.6 | 253.3±7.4 | <0.001 HS |
| 30th Day | 236.0±7.1 | 234.8±7.5 | 227.3±8.6 | 219.2±9.7 | 208.3±13.6 | 196.5±11.5 | 188.8±11.2 | 180.2±11.0 | <0.001 HS |
Values are means ± SD, *Repeated measures ANOVA test, HS= Highly significant
Blood glucose levels seen in sub therapeutic group at fixed time intervals on the days of blood glucose estimation
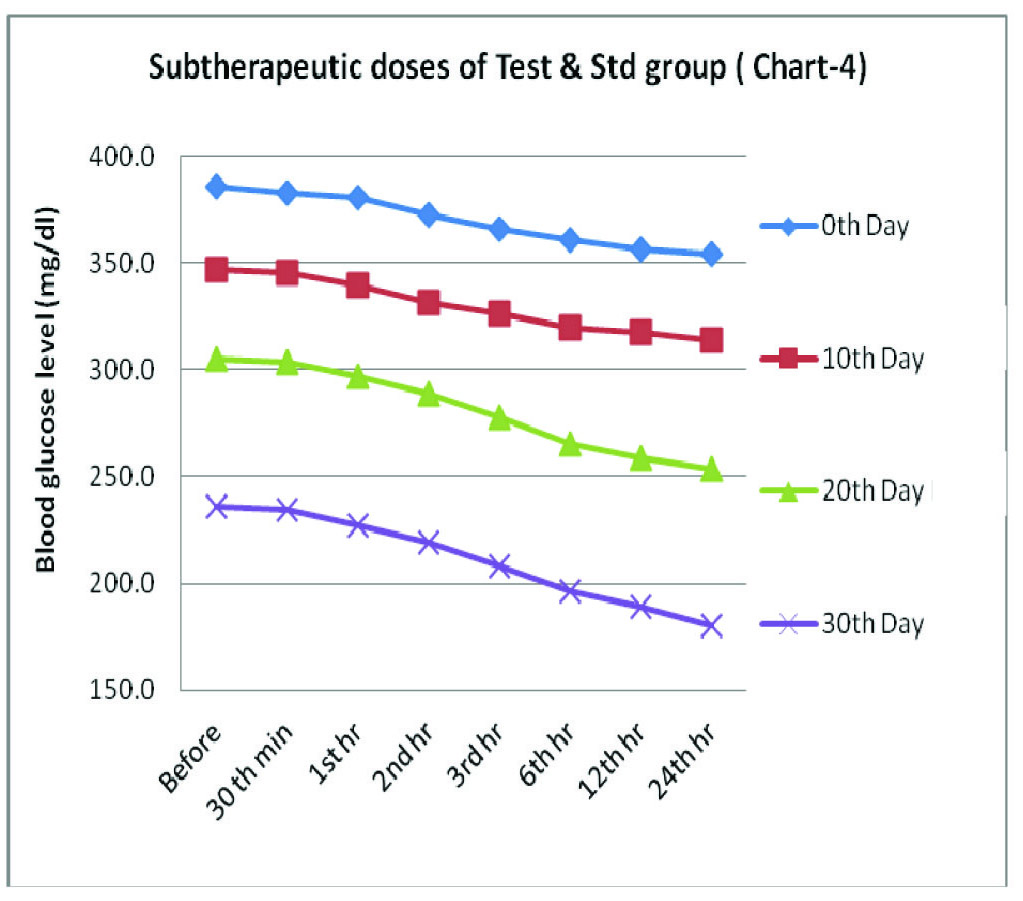
Group 4 which was treated with a combination of sub therapeutic doses of bromocriptine 2 mg/kg body wt and glipizide 1.25 mg/kg body wt, showed highly significant statistics in the fall in blood glucose levels (p<0.001). There was a gradual and a sustained reduction in the blood sugar level, which fell from being 385.7 mg/dl on 0th day to 180.2 mg/dl on 30th day. These observations are quiet comparable with the results of glipizide 2.5 mg/kg body wt treated group.
Discussion
The results indicated that bromocriptine reduced the blood sugar level gradually for a limited period of time and that the reduction would become more prominent on repeated use. The efficacy of bromocriptine alone (4 mg/kg body wt) was not comparable to that of the standard drug glipizide (2.5 mg/kg body wt), but the combination of sub–therapeutic doses of Glipizide 1.25 mg/kg body wt and bromocriptine 2 mg/kg body wt produced an additive effect which was definitely comparable to therapeutic dose of glipizide. Another point which had to be noted was that there was no evidence of any drastic increase in blood sugar levels at any point of time during the experimentation, which indicated their persistent anti-hyperglycaemic effects.
Bromocriptine is unique in that it does not have a specific receptor that mediates its action on glucose and lipid metabolism. Rather, its effects are mediated via resetting of dopaminergic and sympathetic tones within the CNS [11]. There is a large unmet need for new therapies for treating type 2 DM, which reduces fasting and postprandial glucose without increasing insulin levels and which are not associated with weight gain or hypoglycaemia. The quick release formulation of bromocriptine (Cycloset) represents such a therapy [12]. It can thus be specifically indicated as an adjunct to diet and exercise for improving glycaemic control. Type 2 diabetic patients are at a high risk for atherosclerotic CVS complications. There have been reports which have shown beneficial effects of bromocriptine usage in hypertension [13], obesity [14] and dyslipidaemia [15], which in-turn were cause and effect of diabetes. Therefore, bromocriptine can be counted as an anti-diabetic agent that not only improves glycaemia but also provides overall safety in cardiovascular outcomes [16].
The mechanism of action of this agent is unclear; however, its activity as a dopamine D2 receptor agonist seems to provide the primary mechanism for utility in resetting the Circadian rhythm in patients with type 2 DM. Other mechanisms which include an α-1 antagonist, an α-2 agonist and serotonin and prolactin modulators, may also help in explaining bromocriptine’s glucose-lowering effects [17]. The observations of our study showed a definite anti-hyperglycaemic effect with Bromocriptine.
Bromocriptine is known to be associated with nasal stuffiness, nausea, headache, constrictive pericarditis, neuroleptic malignant syndrome and hypotension [18] as various side effects in humans.
Owing to its proposed usage in sub-therapeutic doses due to its anti-diabetic effect, it is thought to produce lesser side effects relatively. However, long term follow ups are required. Further evaluator studies are required to strengthen its safety profile, before implying its wider usage in humans.
Bromocriptine is one of the important drugs which are used in Parkinsonism, hyperprolactinaemia and acromegaly [19], and it is also effective in inducing ovulation [20]. From this study, we can focus on its possible synergistic anti-hyperglycaemic effect with other established anti-diabetic agents like glipizide, in doses which are below normal therapeutic range.
Conclusion
Bromocriptine produces a considerable reduction in the blood glucose but the response with sub–therapeutic doses of combination of bromocriptine and glipizide is quite encouraging. This may reduce the need of full therapeutic doses of individual drugs, thereby reducing the adverse effects and cost of medication. Further clinical trials are required to confirm the above observations. If this is proved beyond doubt, then bromocriptine may become an additional boon for diabetic patients.
Values are means ± SD, *Repeated measures ANOVA test, NS= Non significant
Values are means ± SD, *Repeated measures ANOVA test, HS= Highly significant
Values are means ± SD, *Repeated measures ANOVA test, HS= Highly significant
Values are means ± SD, *Repeated measures ANOVA test, HS= Highly significant
[1]. Mahajan Rajiv, Bromocriptine Mesylate: FDA- approved novel treatment for type-2 diabetesIndian J Pharmacol 2009 August 41(4):197-98. [Google Scholar]
[2]. Musil Beth A, Pharm D CDE, Denise L, Walbrandt Pigarelli, Pharm D BC-ADM, New Drugs for Glycemic Control in Type 2 Diabetes Mellitus. Recently-approved and pipeline drugs for treatment of type 2 diabetesJPSW 2010 Sept/Oct 18 [Google Scholar]
[3]. Petra Kok, Ferdinand Roelfsema, Marijke Frolich, Johannes van Pelt, Stokkel Marcel P M, Meinders A Edo, Hanno Pijl, Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese womenAm J Physiol Endocrinol Metab 2006 June 291:E1038-E43. [Google Scholar]
[4]. Vogel H Gerhard, Wolfgang H Vogel, Bernward A Scholkens, Jurgen Sandow, Gunter Muller, Wolfgang F Vogel, Drug Discovery and Evaluation Pharmacological Assays 2002 2nd EditionSpringer-Verlag Berlag Heidelberg:950 [Google Scholar]
[5]. Gupta SK, Drug Screening Methods 2004 1st editionJaypee publication:307 [Google Scholar]
[6]. Mishra A, Kumari R, Murthy PN, Dash PP, Influence of Atorvastatin on the pharmacodynamics of Glipizide in normal and diabetic ratsDer Pharma Chemica 2010 2(2):101-04. [Google Scholar]
[7]. Jaiswal Dolly, Rai Prashant Kumar, Watal Geeta, Antidiabetic effect of withania coagulans in experimental ratsIndian Journal of Clinical Biochemistry 2009 24(1):88-93. [Google Scholar]
[8]. Ribeiro-de-Oliveira A Jr, Guerra RM, Foscolo RB, Marubayashi U, Reis AM, Coimbra CC, Effects of chronic bromocriptine (CB-154) treatment on the plasma glucose and insulin secretion response to neurocytoglucopenia in ratsJ Endocrinol 1999 Aug 162(2):237-42. [Google Scholar]
[9]. Gosh M N, Fundamentals of Experimental Pharmacology 2005 3rd editionHilton and Company:15 [Google Scholar]
[10]. Kumar Harish VS, Sindhu NR, Patil Rajashri S, Patil Umakant, Anti-hyperglycemic activity of simvastatin alone (therapeutic dose) and combination of simvastatin and glipizide (sub therapeutic doses) on alloxan induced hyperglycemia in albino ratsInt J Pharm Sci Res 2012 3(11):4398-403. [Google Scholar]
[11]. Fronzo Ralph A De, Bromocriptine: A Sympatholytic, D2-Dopamine Agonist for the Treatment of Type 2 DiabetesDiabetes Care 2011 April 34(4):789-94. [Google Scholar]
[12]. Scranton R, Cincotta A, Bromocriptine—unique formulation of a dopamine agonist for the treatment of type 2 diabetesExpert Opin Pharmacother 2010 Feb 11(2):269-79. [Google Scholar]
[13]. Ito Masaharu, Matsuura Kohei, Aiko Akira, Matsui Kazuo, Yoshimura Toshihiro, Okamura Hitoshi, Successful Treatment with Bromocriptine of Hypertension Associated with Polycystic Ovarian DiseaseActa Obstetricia et Gynecologica Scandinavica 1987 66(3):279-82. [Google Scholar]
[14]. Cincotta Anthony H, Meier Albert H, Bromocriptine (Ergoset) Reduces Body Weight and Improves Glucose Tolerance in Obese SubjectsDiabetes Care 1996 June 19(6):667-70. [Google Scholar]
[15]. Zhang Y, Scislowski PW, Prevelige R, Phaneuf S, Cincotta AH, Bromocriptine/SKF38393 treatment ameliorates dyslipidemia in ob/ob miceMetabolism 1999 Aug 48(8):1033-40. [Google Scholar]
[16]. Gaziano J Michael, Cincotta Anthony H, Oconnor Christopher M, Ezrokhi Michael, Rutty Dean, Ma Z J, Scranton Richard E, Randomized Clinical Trial of Quick-Release Bromocriptine Among Patients With Type 2 Diabetes on Overall Safety and Cardiovascular OutcomesDiabetes Care 2010 33:1503-08. [Google Scholar]
[17]. Kerr JL, Timpe EM, Petkewicz KA, Bromocriptine mesylate for glycemic management in type2 Diabetes MellitusAnn Pharmacother 2010 Nov 44(11):1777-85. [Google Scholar]
[18]. Evans Joseph L, Rushakoff Robert J, Oral Pharmacological Agents for Type 2 DiabetesENDOTEXT 2010 December [Google Scholar]
[19]. Brunton LL, Lazo JS, Parker KL, Goodman and Gilman’s The Pharmacological Basis of Therapeutics 2005 11th editionNew York, USAMc Graw Hill Publications:1500 [Google Scholar]
[20]. van der Steeg H J, Bennink H J T Coelingh, Bromocriptine for induction of ovulation in normoprolactinaemic post-pill anovulationThe Lancet 1977 March 5 309(8010):502-04. [Google Scholar]