Background: Carvedilol is a commonly used drug in hypertension, congestive heart failure in diabetics. It has moderate calcium channel blocking property in addition to α1 and non selective β antagonistic activity. Though some studies bring forth the beneficial effects of Carvedilol in cardiovascular comorbidities in diabetes, there is no consensus on its effects on glycaemic levels.
Aims: To evaluate the effect of oral Carvedilol administration for 5 days on blood glucose levels in normal albino rats through Oral Glucose Tolerance Test.
Material and Methods: Twelve adult albino rats of either sex weighing between 150 – 200 g were selected from central animal facility and randomly divided into 2 groups – Control [Distilled water (1ml/rat orally)] and Test (0.8mg/kg body weight orally) and the respective drugs were administered over 5 days. Following overnight fasting, on the fifth day 1 hour after the last dose of the respective drug, OGTT was performed. The CBG (Capillary Blood Glucose) levels were measured at 0 min, glucose (2g/kg body weight) dissolved in water was administered to all the rats orally. The blood sample from tail vein (obtained by tail snipping) at 60 and 150 minutes were analysed for CBG levels using a standardized glucometer.
Statistical Analysis: Data was presented as Mean ± SEM. One way ANOVA, independent samples t-test, non–parametric tests, percentages and cross tabs were used in the analysis of data within the same group and between different groups when required.
Results: Carvedilol group showed higher CBG levels at all time intervals of OGTT as compared to the Control group i.e., 0, 60 and 150 minutes, the highest being (103.8±5.029 )mg/dl at 60 minutes and was statistically significant. Carvedilol group however showed lesser inter–interval variation compared to the Control group at the same time intervals respectively but was statistically insignificant.
Conclusions: Carvedilol has hyperglycaemic potential when given orally for 5 days in normal albino rats. Though it may be beneficial in diabetics for various comorbid conditions, the glycaemic control may worsen during its use in subjects with prediabetes, diabetes, high risk diabetes.
Introduction
Diabetes mellitus is a chronic disease which poses a significant challenge for pharmacologists and physicians worldwide. With an incidence of 285 million (90% with type 2 diabetes) [1] and 344 million with impaired glucose tolerance worldwide it requires vigilant attention especially in Asia and Africa [2,3]. India is the diabetes capital of the world [4].
Diabetes mellitus coexisting with hypertension is a very common occurrence with 90% of the coexisting diabetes being type 2. Thiazide diuretics (proven), Calcium channel blockers (controversial role) have been known to cause hyperglycaemia in patients irrespective of whether they were in normoglycaemic/impaired fasting glucose/impaired glucose tolerance / diabetic state before treatment with these antihypertensives. The incidence rate of such new onset diabetes is as high as 2% in treated hypertensives [5]. Also, cardiovascular diseases like coronary heart disease, congestive heart failure are major causes of morbidity and mortality in diabetes subjects [6,7]. Insulin resistance in type 2 diabetes mellitus leading to glucotoxicity may also worsen coronary artery disease [7]. Better control of glycaemic levels have resulted in a reduction in the risk of cardiovascular morbidity or mortality in diabetics [8].
Insulin secretion is influenced by the changes in intracellular calcium levels which are in turn modulated by the movement of calcium into the cells through the various voltage gated calcium channels( L and T type ) of the pancreatic beta cell membrane which are in turn significantly dependent on glucose metabolism reflected by the blood glucose levels. Thus, it can be safely suggested that drugs used in the management of hypertension, cardiac failure etc in diabetics or normoglycaemic individuals which may induce hyperglycaemia may cause significant cardiovascular effects indirectly through their action on insulin secretion [9,10].
Carvedilol is a drug used in hypertension, angina and congestive heart failure. It is β1, β2 antagonist(onset in 1 hour), α1 antagonist( onset in 30 minutes) and on oral administration it is rapidly absorbed and reaches peak plasma concentration within one to 2 hours. At higher doses, it has calcium channel blocking property of moderate potency (blocks L-type voltage gated channels) [11,12] but the effect of this blockade on blood glucose levels is a scantily explored domain [13,14]. Some studies reported beta blocking induced hyperglycaemia contradicting the ideas of its vasodilating property inducing hypoglycaemia by improving insulin sensitivity [15,16,17].
Oral Glucose Tolerance Test (OGTT) is performed to assess glucose tolerance and it is indirectly indicative of insulin sensitivity and beta cell function including insulin secretion. The results obtained in rodents are quantifiable easily and are applicable to human beings [18].
Though the beta blocking effect may predispose Carvedilol to induce and mask hypoglycaemia, its calcium channel blocking property at higher doses may be the factor tipping the balance in favour of it causing hyperglycaemia especially in prediabetics and diabetics. Hypertension, a common indication for Carvedilol, has a higher incidence in diabetics [19]. With this background, this study was conducted to assess the effect of Carvedilol on blood glucose levels in normal albino rats in an attempt to explain the complex interaction of the various actions (beta blocker, alpha 1 blocker, calcium channel blocker) of Carvedilol and its collective effect on blood glucose levels.
Methods
a) Test systems used
Animals
Adult albino rats of either sex weighing between 150 – 200g were randomly selected from central animal facility, JSS Medical College, Mysore, India. Animals which were diseased or pregnant were excluded. They were housed in groups of 3 with access to food and water as usual at 25°C and were acclimatized to these surroundings for 5 days before the study was undertaken. Tap water and pelleted standard diet (Amruth distributors supplied through Kamadhenu agencies, Bangalore) were available ad libitum.
b) Drugs and chemicals reagents used in the study
Glucose 2g/kg body weight dissolved in water–given orally
Carvedilol tablets procured from the hospital pharmacy and dissolved in distilled water–0.8 mg/kg body weight–given orally
Distilled water–1ml/rat–given orally
c) OGTT (Oral Glucose Tolerance Test)
OGTT is a more physiological method of assessing the glucose induced insulin secretion and its effect on glycaemic control applicable when performed in normal rats. This study used OGTT for normoglycaemic rats with some modifications to the standard method described by Du vigneaud and Karr, 1925.
At the end of acclimatization period, 12 albino rats were selected and randomly divided into 2 groups – Control and Test. Distilled water was given to the Control group and Carvedilol was given to the test group over 5 days via oral gavage tube. Following overnight fasting on the fifth day, 1 hour after the last dose of the respective drug, OGTT was performed. The CBG (Capillary Blood Glucose) of all the rats were measured at 0 hour. Following this, glucose (2g/kg body weight) dissolved in water was administered to all the rats orally using gavage tube. The blood sample from tail vein (obtained by tail snipping) was then assessed for glucose levels at 60 and 150 minutes using a standardized glucometer. (“One Touch” company).
Ethics
The study protocol was approved by Institutional Animal Ethics Committee, JSS Medical College, Mysore, India.
Statistics
The data was presented by calculating the mean and SEM of the outcome parameters. Statistical comparisons were done by One way or repeated measures Analysis of Variance (ANOVA) for CBG values at different time intervals within the same group followed by independent samples t–test to see the difference between the two groups when required. Percentages and cross tabs were used as necessary. Tests of significance were carried out at 5% level and p value <0.05 was considered as statistically significant. All the statistical calculations and graphs were analysed using the Graph Pad Prism 5.00 version for Windows.
Results
Carvedilol group showed elevated blood glucose levels throughout the OGTT compared to Control with the highest elevation in CBG being observed at 0 minute (33.67±13.91) which was statistically significant (p=0.005) [Table/Fig-1 and 2].
CBG (Capillary blood glucose) levels in Control group, Carvedilol group and Difference between Control and Carvedilol groups in mg/dl and percentage
| Time interval during OGTT | Blood glucose concentration in mg/dl (mean±SEM) | % increase in CBG levels in Carvedilol group |
|---|
| Control group (n=6) | Carvedilol group (n=6) | Rise in Carvedilol group compared to Control group |
|---|
| 0 min | 54.5 ± 1.962 | 88.17 ± 15.35 | 33.67 ± 13.91 | 28.5 |
| 60 min | 94.83 ± 4.586 | 103.8 ± 5.029 | 9 ± 1.807 | 8.97 |
| 150 min | 67.67 ± 2.860 | 85 ± 4.235 | 17.33 ± 5.981 | 17.33 |
The CBG levels in mg/dl of Control and Carvedilol at 0, 60, 150 minutes of OGTT respectively compared with each other
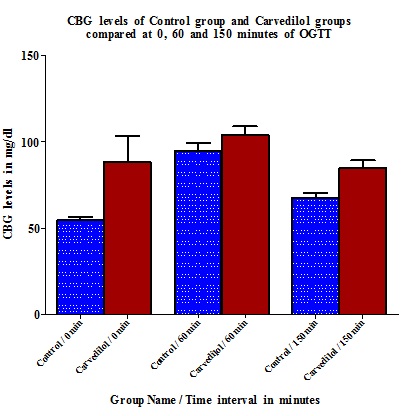
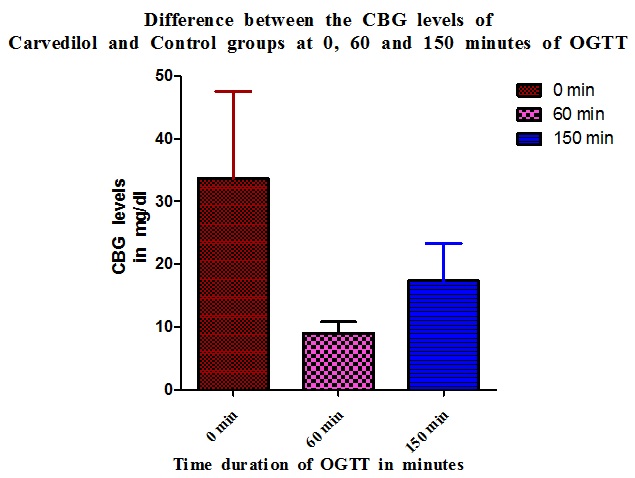
The peak difference in CBG level elevation in Carvedilol group compared to Control is at 0 minute (28.5%) and the least difference is at 60 minute interval (8.97%) of OGTT [Table/Fig-1 and 3].
The increase in CBG levels of Carvedilol group compared to that of Control group at respective time intervals i.e. 0, 60, 150 minutes of OGTT
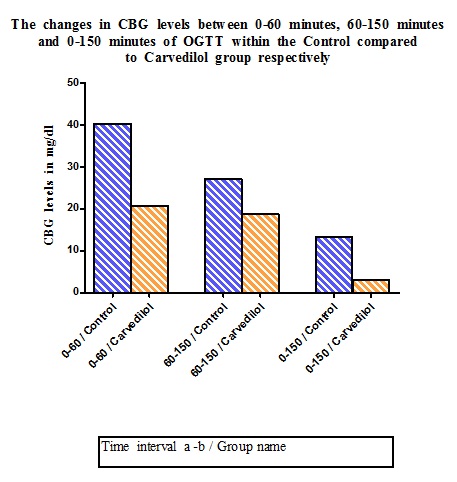
In the Carvedilol group, the CBG levels remained consistently higher than Control group but with lesser changes in CBG levels through different intervals i.e. 0–60 minute, 60–150 minute or 0–150 minute. However, the higher inter–interval differences seen in the Control group was not statistically significant (p = 1.000; 0.421;0.054 respectively) [Table/Fig-4 and 5].
Change in CBG of the groups between various time intervals of OGTT
| Sl. No | Time interval | Change in CBG values (mg/dl) |
|---|
| Control | Carvedilol |
| 1. | 0–60 min | 40.33 ± 6.280 | 20.8 ± 9.272 |
| 2. | 60–150min | 27.16 ± 6.085 | 18.8 ± 5.433 |
| 3. | 0–150 min | 13.17 ± 1.887 | 3.17 ± 1.25 |
The alteration in CBG levels during the progression of OGTT from one measurement interval to the other within Control and Carvedilol group are analyzed separately and the differences of respective inter- interval variation are compared between the two groups
Time interval a-b – i.e. From time interval ‘a’ to time interval ‘b’ in the respective group
0-60 minute – time duration of 1 hour- indicates CBG level elevation from basal level to peak level
60-150 minute – time duration of 1 ½ hours- indicates CBG level elevation from peak level to back to basal level
0-150 minute – time duration of 2 ½ hours- indicates CBG level elevation between pre-OGTT basal and end-OGTT back to basal level
The analysis comparing all the intervals blood glucose levels in both the Control and Carvedilol groups revealed that there was statistically significant differences between Carvedilol 60 and Control 150 minute; Control 60 and Control 0 minutes; Carvedilol 60 and Control 0 minutes; Carvedilol 0 and Control 0 min CBG values. The highest CBG levels amongst all was observed at 60 minutes of OGTT in the Carvedilol group (103.8±5.029) [Table/Fig-1 and 6].
Difference in CBG levels at all the time intervals of OGTT of Carvedilol group compared to that of the Control group.
| Sl. No. | OGTT time interval of Carvedilol – OGTT time interval of Control (T - C) | Difference in CBG values (mg/dl) |
|---|
| 1. | 0-0 min | 28.5 |
| 2. | 0- 60 min | 11.83* |
| 3. | 0- 150 min | 15.33 |
| 4. | 60- 0 min | 49.3 |
| 5. | 60-60 min | 8.97 |
| 6. | 60-150 min | 36.13 |
| 7. | 150- 0 min | 30.5 |
| 8. | 150-60 min | 9.83* |
| 9. | 150 -150min | 17.33 |
(*- CBG of the Carvedilol is lesser than that of the Control at the time intervals compared)
The analysis of the difference in CBG levels at all intervals of OGTT between the two groups revealed that Carvedilol group showed a higher CBG level as compared to all intervals of Control group except the 0 minute and 150 minute. The 0 minute and 150 minute CBG levels of the Carvedilol group were lower than the 60 minute Control group values in both the instances even though the 60 minute value of Carvedilol group was higher than the 60 minute Control group values. The maximum difference in CBG levels were noted at 60 minute of Carvedilol to 0 minute of Control (49.3mg/dl) [Table/Fig-6].
Discussion
Oral glucose tolerance test is an effective way to assess the effect of a drug on blood glucose levels as it mirrors the glucose level changes that occur following a meal which is the most important determinant of insulin secretion and is used frequently to assess the glucose tolerance.
In this study, Carvedilol, administered at the dose of 0.8mg/kg body weight for 5 days, showed higher CBG levels at 0 minute of OGTT and the highest CBG levels (103.8±5.029) at 60 minutes of OGTT when compared to the Control group. Although Carvedilol group showed a higher level of CBG at all intervals of OGTT as compared to the Control group, statistical significance was seen at 0 hour CBG levels of both when compared at individual intervals of each group. Thus, Carvedilol caused a consistent elevation in basal blood glucose levels which increased further during the OGTT following oral glucose administration. The inter-interval rise between 0–60 and 60–150 minute of OGTT in the Carvedilol group are not being as high as the Control group may be due to the baseline levels of CBG in the Control group being lower and thus with more potential space for the blood glucose levels to increase than the already high basal CBG levels seen in the Carvedilol group. However, the peak blood glucose levels expected in OGTT is at 60 minutes and this was observed to be true in the Carvedilol group with the highest CBG levels being observed at 60 minutes of OGTT.
The higher inter–interval difference in CBG levels in the Control group being statistically insignificant highlights the concept that even though there is no significant increase in blood glucose levels through different intervals of time in OGTT, there is definitely no lowering when compared to control i.e., the blood glucose levels of Carvedilol at individual intervals are always higher than the Control group. This proves that Carvedilol group causes a baseline hyperglycaemia even at 0 minute and 150 minute of OGTT when the blood glucose levels are supposed to be near normal which may not be as high as the peak Control group CBG levels but is higher than all other blood glucose levels attained in the Control group. This underlines the hyperglycaemic effects of Carvedilol.
The above findings are indicative of the effect of Carvedilol as an agent inducing hyperglycaemia in normal albino rats. Considering the fact that OGTT is an indicator of glucose tolerance in general [18], it can be safely assumed that Carvedilol worsens the existing hyperglycaemia in diabetics and prediabetics when used for various indications in such subjects. However, this assumption needs to be tested on hyperglycaemic rats in the future. Since the non–selective β blockers apart from Carvedilol actually induce or mask the signs of hypoglycaemia, the findings of this study give credibility to the hypothesis generated at the beginning of this study i.e. the moderately potent calcium channel blocking property of Carvedilol will cause hyperglycaemia above and beyond the effect on blood glucose caused by its α1 and β antagonism.
The blockage of L–type voltage gated calcium channels in beta cells of by Carvedilol will fail to allow intracellular transfer of Calcium ions and the resultant processes within the beta cells ultimately resulting in the release of insulin into the blood causing hypoglycaemia [10] thus resulting in hyperglycaemia induced by Carvedilol administered for 5 days. The other possible mechanisms of hyperglycaemia induced by Carvedilol are by directly decreasing insulin secretion and also decreasing glucagon evoked release of insulin which however may be counteracted by hypoglycaemic effects via action on liver, muscle. (β2 blocakade mediated) [20] which need to be explored in future studies. This also contends with the hypoglycaemic effects of Carvedilol reported in some studies [15,16,17]. Whether this hyperglycaemia is severe enough to induce glucotoxicity and worsen the outcomes of the comorbidities in individuals with diabetes/ prediabetic state when Carvedilol is used needs to be analyzed. The findings of this study also highlights the need to revisit the notion that Carvedilol improves the co-morbidities in diabetics [17] by beneficial effect on blood glucose levels. Though the beneficial effects of Carvedilol when used in diabetics for various indications is not questioned [17], the notion that such a beneficial effect is due to improvement in glucose tolerance, is questioned by the findings of this study.
This is of concern when considering Carvedilol for any indication in diabetics for its antioxidant property on the basis of some clinical trials which have claimed it to be comparatively better than certain selective β1 blockers [8]. This study also explains the absence of hypoglycaemic effects with the use of Carvedilol which is observed with the use other non selective β blockers.
To conclude, Carvedilol, an important drug used in the treatment of hypertension, angina, congestive cardiac failure especially in diabetic patients, produces hyperglycaemia as evidenced by the hyperglycaemia inducing effects on normal albino rats observed in this study. This may adversely affect the glycaemic control in prediabetics/diabetics/high risk diabetics when used for various indications.
Acknowledgement
We would like to thank our institution for permitting and providing the necessary assistance for the study.
Limitations
Multiple doses of Carvedilol have not been tested since higher doses have a more evident L–type voltage gated calcium channel blocking effect.
Since the hypothesis stressed upon the calcium channel blockade effect of Carvedilol, the study has been conducted regarding OGTT induced changes in blood glucose which reflects upon glucose induced and thus calcium mediated insulin secretion which is more evident following a glucose load.
The other possible mechanisms of hyperglycaemia like beta blockade have not been explored directly since the emphasis was on the calcium channel , glucose load and Carvedilol’s effect on the same.
(*- CBG of the Carvedilol is lesser than that of the Control at the time intervals compared)
[1]. Melmed S, Polonsky KS, Reed Larsen PR, Kronenberg HM, Williams Textbook of Endocrinology 2011 12th edPhiladelphiaElsevier/Saunders:1371-1435. [Google Scholar]
[2]. Wild S, Roglic G, Green A, Sicree R, King H, “Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030”Diabetes Care 2004 27(5):1047-53. [Google Scholar]
[3]. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives Lei Chen, Dianna J. Magliano & Paul Z. ZimmetNature Reviews Endocrinology 2012 8:228-36. [Google Scholar]
[4]. Gale, Jason (November 7, 2010). “India’s Diabetes Epidemic Cuts Down Millions Who Escape Poverty”. Bloomberg. http://www.bloomberg.com/news/2010-11-07/india-s-deadly-diabetes-scourge-cuts-down-millions-rising-to-middle-class.html. Retrieved 8 June 2012 [Google Scholar]
[5]. Verdecchia Paolo, Reboldi Gianpaolo, Angeli Fabio, Borgioni Claudia, Gattobigio Roberto, Filippucci Lucia, Adverse Prognostic Significance of New Diabetes in Treated Hypertensive SubjectsHypertension 2004 43:963-69. [Google Scholar]
[6]. Rubler S, Dlugash J, Yucheogly Y, New types of cardiomyopathy associated with diabetic glomerulosclerosisAm J Cardiol 1972 30:595 [Google Scholar]
[7]. Simone GD, Devereux RB, Chinali M, Lee ET, Galloway JM, Barac A, Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart StudyJ Hypertens 2010 28(2):353-60. [Google Scholar]
[8]. Bakris GL, Fonseca V, Katholi RE, McGill JB, Messerli FH, Phillips RA, Metabolic Effects of Carvedilol vs Metoprolol in Patients With Type 2 Diabetes Mellitus and Hypertension A Randomized Controlled TrialJAMA 2004 292(18):2227-36. [Google Scholar]
[9]. VonLewinski D, Bruns S, Walther S, Kögler H, Pieske B, Insulin causes [Ca2+]i-dependent and [Ca2+]i-independent positive inotropic effects in failing human myocardiumCirculation 2005 111(20):2588-95. [Google Scholar]
[10]. Satin LS, Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of LangerhansEndocrine 2000 13(3):251-62. [Google Scholar]
[11]. Feuerstein GZ, Ruffolo Jr RR, Carvedilol, a novel vasodilating beta-blocker with the potential for cardiovascular organ protectionEuropean Heart Journal 1996 17:24-29. [Google Scholar]
[12]. Nichols AJ, Sulpizio AC, Ashton DJ, Hieble JP, Ruffolo, Jr. RR, In vitro Pharmacologic Profile of the Novel Beta-Adrenoceptor Antagonist and Vasodilator, CarvedilolPharmacology 1989 39:327-36. [Google Scholar]
[13]. Vanderhoff BT, Ruppel HM, Amsterdam PB, Carvedilol: The New Role of Beta Blockers in Congestive Heart FailureAm Fam Physician 1998 58(7):1627-34. [Google Scholar]
[14]. Byington RP, Craven TE, Furberg CD, Pahor M, Isradipine, raised glycosylated haemoglobin, and risk of cardiovascular eventsThe Lancet 1997 350(9084):1075-76. [Google Scholar]
[15]. Whalen KL, Stewart RD, Pharmacologic Management of Hypertension in Patients with DiabetesAm Fam Physician 2008 78(11):1277-82. [Google Scholar]
[16]. Kim YS, Kim SY, Bae JH, Nah DY, Rhee MY, Lee MM, Telmisartan Versus Carvedilol in Hypertensive Patients with Metabolic Syndrome: Effects on Blood Pressure, Arterial Stiffness, Blood Glucose, and Lipid MetabolismJ Korean Soc Hypertens 2010 Dec 16(4):44-53. [Google Scholar]
[17]. Lleva RR, Inzucchi SE, Glucose, Blood Pressure, and Cardiovascular RiskCirc Cardiovasc Qual Outcomes 2012 5:145-47. [Google Scholar]
[18]. Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C, Oral Glucose Tolerance Test Minimal Model Indexes of β-Cell Function and Insulin SensitivityDiabetes 2001 50:150-58. [Google Scholar]
[19]. Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL, Hypertension and Antihypertensive Therapy as Risk Factors for Type 2 Diabetes MellitusN Engl J Med 2000 342:905-12. [Google Scholar]
[20]. Tripathi KD, Adrenergic system and drugsEssentials of Medical Pharmacology 2010 6th edIndiaJaypee Brothers Medical Publishers (P) Ltd:122 [Google Scholar]