Anti Mullerian Hormone: A Potential Marker for Recruited Non Growing Follicle of Ovarian Pool in Women with Polycystic Ovarian Syndrome
P Saikumar1, VS Kalai Selvi2, K Prabhu3, Prasana Venkatesh4, Prashanth Krishna5
1 Professor, Department of Physiology, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India.
2 Professor, Department of Biochemistry, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India.
3 Associate Professor, Department of Anatomy, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India.
4 MBBS Student, Sree Balaji Medical College and Hospital, Chennai, Tamil Nadu, India.
5 MBBS Student, Sree Ramachandra Medical College, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. P. Saikumar, Professor and H.O.D, Department of Physiology, Sree Balaji Medical College and Hospital, No. 7, CLC Works Road, New Colony, Chromepet, Chennai-600044, Tamil Nadu, India.
Phone: 98400 98098;
E-mail: drpsai@gmail.com
Background: Polycystic ovarian disease is one of the most common causes of infertility in women of reproductive age. Anti– mullerian hormone (AMH), a member of transforming growth factor (TGF) family which is secreted by granulosa cells of growing follicle, is found to be increased to three to four fold in Poly Cystic Ovarian Syndrome (PCOS) patients as evidenced by previous studies. But the level of AMH in relation to the infertile status of PCOS was not studied yet. The present study was focused to determine the discriminative power of AMH in infertility subjects with regular cycles and infertility subjects associated with PCOS.
Methods: The subjects under study were one hundred and twenty infertile women of age group ranging from 27–35 years. Subjects, were further divided into sixty infertile with regular cycles as controls (Group1) and sixty infertile subjects with PCOS as cases (Group 2). Hormones like FSH, E2 and AMH were assayed for all the subjects. Mean and student t– test for all hormones were compared between controls and cases. The diagnostic power of AMH pertaining to sensitivity and specificity was evaluated by Receiver operating characteristic (ROC) curve.
Results: Serum AMH level were two fold higher in PCOS patients than in controls. The mean value of AMH also shows a test of significance between the two groups. The area under the receiver operating characteristic curve for the AMH assay was 0.95 in infertile group when 3.34ng/ml was used as cut off point indicating its better discriminative power and good diagnostic potency. Setting the AMH value at 3.34ng/ml sensitivity, specificity,Positive Predictive Value(PPV) and Negative Predictive Value(NPV) were observed 98% ,93%, 93% and 98% respectively.
Conclusion: The diagnostic potency of Area Under Curve (AUC) for AMH in infertile subjects reflects that AMH is a potential marker for recruited non growing follicles rather than a simple marker for ovarian reserve as it is predominantly produced by small follicles rather than a simple marker for ovarian reserve.
Ovarian Reserve, PCOS, AMH, AUC, Non growing follicle, Infertility
Background
The primary function of the human ovary is the production of sex steroid hormones and gametes. At around 20 weeks of foetal development, the female gamete forms primordial follicles and with the onset of menarche, the follicles grow in size. Recruitment of follicles for the ovulation process continues until the primordial follicle pool is exhausted, resulting in menopause in women.
The size of the primordial follicle stocks is difficult to measure directly and studies have suggested that the number of growing follicles is correlated to the size of primordial follicle stock from which they are recruited [1, 2]. A marker is required to ascertain the transition from the primordial follicle to the growing follicle, which reflects the qualitative and quantitative assessment of the ovarian reserve. Transvaginal ultrasonography measurement of antral follicular count (AFC) and ovarian volume (OV) indicate and reflect the size of primordial follicle pool. The ovarian volume (OV) indirectly reflects the ovarian reserve. But, it is very difficult to define the normal OV size in the reproductive age group. Hormonal parameters such as FSH, E2, Inhibin and Anti Mullerian Hormone (AMH) have been proposed to serve as predictors of ovarian reserve.
Measuring Day 3 FSH and E2 is also an indirect assessment of the size of follicle cohort. In conditions which are associated with irregular cycles such as PCOS, it is very difficult to predict the appropriate time which is required for measuring FSH and E2. In regularly menstruating women who are between the ages of 24 and 50 years, there is no difference in the basal oestradiol level with respect to age [3].
Antral follicular count is a better marker than age and FSH for distinguishing between good and poor pregnancy prospects in patients of a little higher age group [4]. According to the data of a single study, a poor response to ovarian stimulation can be predicted with the help of AFC, which has a sensitivity of 0.89, a specificity of 0.39 and a positive likelihood ratio of 1.45 [5]. As the ovarian volume and hormonal parameters have poor reliability for defining ovarian reserve in PCOS, the only marker which can directly assess the ovarian reserve is the anti mullerian hormone.
AMH is produced by the granulosa cells of the recruited follicles until they become sensitive to FSH [6]. AMH has been identified as a regulator of the recruitment process, which prevents the depletion of all primordial follicles at once [7]. Indeed, increased AMH levels in serum were found in PCOS patients for whom the number of pre antral and small antral follicles were 2-3 fold higher as compared with those of normal ovaries [8–10].
PCOS is the most common endocrine disorder in women of reproductive age group and it is also one of the challenging issues which causing infertility. In this study, we focused on the threshold value of AMH, which had high specificity and sensitivity as a biological marker for discriminating PCOS from controls in normo gonadotrophic infertile patients.
Methods
This study was carried out over a period of one year, between Nov 2010 and Nov 2011, on patients who attended the Prashanth Fertility Centre and Sree Balaji Medical College and Hospital. The study was approved by the institutional ethical committee.
The participants were informed about the study and their consents were received. The subjects who were under study were one hundred and twenty women of age group of 27–35 years. Sixty infertile subjects with regular cycles were considered as controls and they were categorized as Group 1 and sixty infertile subjects with PCOS were considered as cases and they were categorized as Group 2 respectively.
Controls in Groups 1: Menstrual cycles were considered to be regular if they occurred between 28-35 days and if ovaries appeared normal on transvaginal ultrasonography.
Cases in Groups 2: All PCOS subjects with oligomenorrhoea, who were included in this study, were those who fulfilled any two of the following 2003 revised Rotterdam diagnosis criteria:
Prolonged oligo – ovulation (6 or fewer menses per year or anovulation)
Clinical hirsutism which was defined by a Feriman Gallwey score of >7, [acne, androgenic alopecia and or biochemical signs which were produced by testosterone for hyper Androgenism (HA)].
A PCO morphology on ultrasound examination which revealed >10 cysts which were 2 to 9 mm in diameter, which were distributed evenly around the ovarian periphery, with an increased amount of stroma.
Infertile women with regular cycles (Growth–1) and those with irregular cycles with PCOS (Growth–2) were selected from the Prasanth Fertility Centre and Sree Balaji Medical College and Hospital.
Exclusion criteria: Subjects with Diabetes Mellitus were excluded from the study.
Ovarian volume and antral follicular count were measured by ultrasound, and hormones such as FSH, E2 and AMH were measured on day 3 after the last menstrual period for all the subjects. Serum AMH was assayed by using the AMH/ MIS Enzyme Linked Immuno Sorbent Assay (ELISA) kit (Immunotech-Beckman, Marseilles, France). The assay sensitivity was 0.7 pmol/l. The intra and inter assay coefficients of variation were 5.3% and 8.7% respectively. FSH was analyzed by using MONOBIND (ELISA), Inc, and E2 was measured by using Biosource, Beljium (ELISA).
Statistical Analysis
Continuous data have been shown as mean + SD. Differences in AFC, AMH and levels of hormones such as FSH and E2 between PCOS and regular cycle subjects were assessed by using Student’s t-test. Receiver operating characteristic (ROC) curves were constructed for AMH to assess the diagnostic test performance, i.e. the capacity to discriminate between controls and patients with PCOS for AMH. A curve with sensitivity in Y–Axis ( Sensitivity) against X–axis (1–specificity) was plotted at 75th and 90th percentile value of AMH for regular cycles in infertile groups and area under curve was computed. AUC represented the probability of correctly identifying controls and patients with PCOS. An AUC value of 0.5 indicated that the test had no discriminative power, and a value of 1.0 indicated that the test had perfect discrimination.
Results
The mean values of the general characteristic features such as age and BMI, and of hormonal parameters such as FSH, E2 and AFC have been depicted in [Table/Fig-1]. As it was expected, patients with PCOS showed higher BMI and E2 values, and low levels of FSH than controls. Likewise, the mean value of follicles of sizes 2-9 mm was two fold higher in PCOS and it shows statistically significant values between PCOS and controls.
Test of significance of the variables between PCOS and regular cycle subjects in Group 1 and Group 2
| Screening Parameters | Infertile Subjects |
|---|
| Group 1 controls | Group 2 PCOS | p value |
|---|
| Age (years) | 30.9 ± 2.79 | 33.4 ± 4.1 | 0.447 |
| BMI(Kg/m2) | 25.0 ± 3.3 | 27.5 ± 2.65 | 0.23 |
| FSHmIU/mL) | 6.04 ± 1.7 | 5.6 ± 2.1 | <0.01 |
| E2(m mol/mL) | 127.8 ± 38.9 | 160.5 ± 54.9 | 0.157 |
| 2-9mm follicle no | 12 ± 6.8 | 26.3 ± 24 | <0.001 |
| AMH(ng/mL) | 2.27 ± 0.806 | 4.38 ± 2.24 | <0.001 |
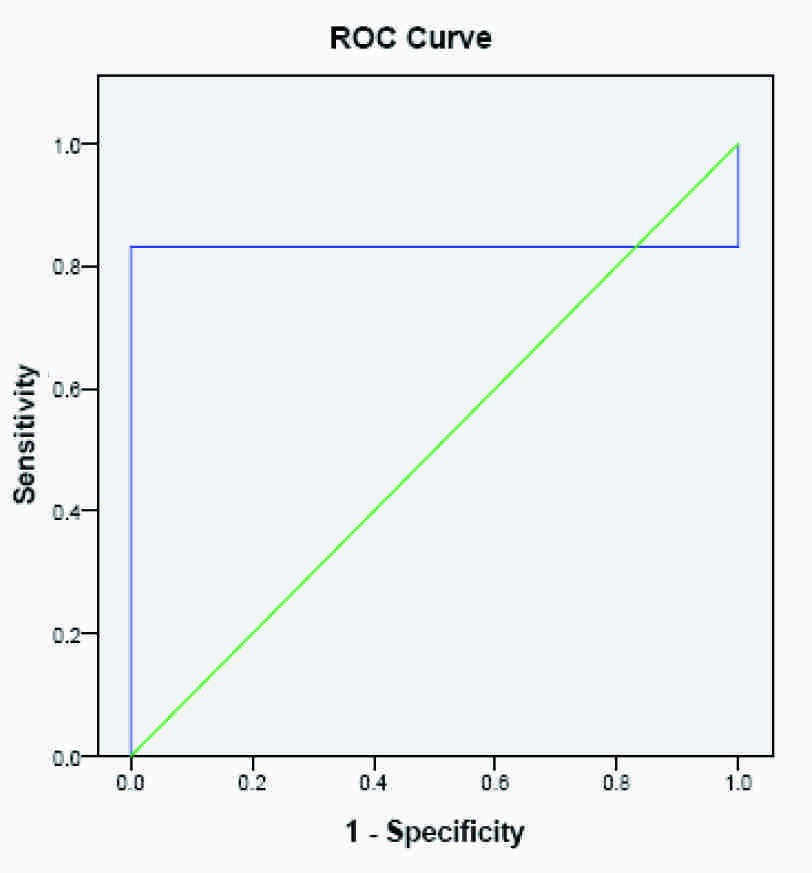
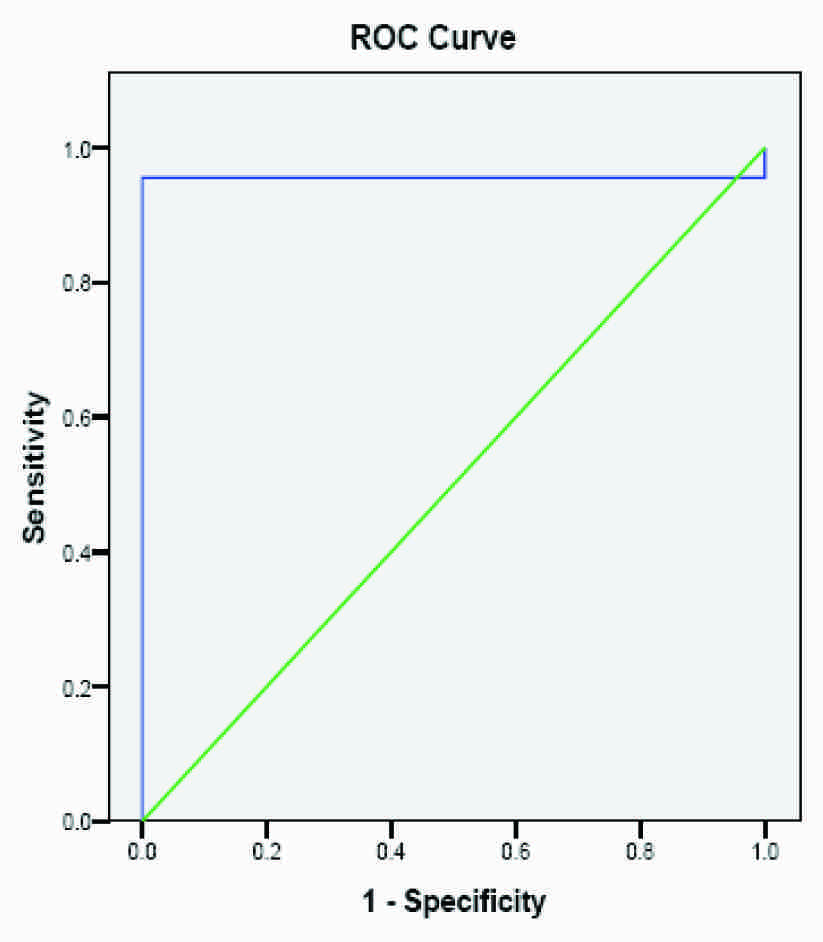
The test performance for the diagnostic potency of AMH, as quantified by the AUC for PCOS in infertile patients, has been shown in [Table/Fig-2]. The area under the ROC curve for AMH reached a value of 0.831 with a cut off value of 2.93 ng/ml and this value was slightly higher than the 75th percentile of controls, as has been shown in [Table/Fig-3]. When the value of 90th percentile (3.34 ng/ml) was taken as cut off point, the area under the ROC curve reached a value of 0.956, as has been shown in [Table/Fig-4]. The cut off values of serum AMH levels were also analyzed in terms of sensitivities, specificities, positive predictive value (PPV) and negative predictive value (NPV). [Table/Fig-2] has interpreted that the best compromise between specificity (93%) and sensitivity (98%) was obtained with a cut off value of 3.34 ng/ml. This value was slightly higher than the 90th percentile of controls, which predicted that AMH reflected the number of non growing follicles rather than ovarian reserve in the ovary.
shows AMH threshold level of 5th – 75th, 5th–90th percentile value in regular cycle subjects as cutoff points to determine AUC, sensitivity, specificity, PPV and NPV.
| AMH Threshold Level | AUC | Sensitivity | specificity | PPV | NPV |
|---|
| 1.61 – 2.93 | 0.831 | 100 | 80 | 83 | 100 |
| 0.87 – 3.34 | 0.956 | 98 | 93 | 93 | 98 |
ROC Curve for AMH using 2.93 as cut of point
ROC Curve for AMH using 3.34 as cut of point
Discussion
In this study, we investigated as to whether AMH measurement could be a valuable diagnostic marker of PCOS. Previous studies which have been reported in the literature have suggested that AMH was a potential marker of ovarian reserve. Antral follicular count was closely related to AMH in infertile women, as was demonstrated by Fanchin et al., [11]. Several workers reported an increase of 2 to 4 fold of serum AMH levels in PCOS patients [12, 8] and they also observed a close association between AMH levels and pre–antral follicular count. Therefore, data from previous studies have shown that AMH could be the biological marker of an early antral follicular number, in normo–ovulatory cycles and in PCOS women, but that it underlined its robustness as a diagnostic marker for discriminating PCOS women from controls among infertile women.
Screening tests, diagnostic tests and prognostic tests are the different kinds of tests which are needed for obtaining additional information for assessing the ovarian reserve status. A poor ovarian reserve does not fulfill the criteria of a disease. The probability of conception was still questionable, as to how good these ovarian reserve tests had sensitivity and specificity, as was addressed by Jain [13]. Other factors such as endometriosis, an increased body mass index, and male factor could also confound the accuracy of the test, while sub fertility was dealt with.
Basal serum FSH, AFC, oestradiol or OV levels do not fulfill the criteria of a good screening test for assessing the ovarian reserve. Most of them will diagnose poor ovarian reserves, but only at the extreme range of values and these values are yet to be standardized. AMH, which is produced by the cells of the recruited follicles, is the only marker of ovarian reserve that can be tested in follicular as well as luteal phase, although the threshold levels in both phases for regular cycles and PCOS need to be standardized. AMH levels have been found to be two or three times higher in PCOS women [8–10], thus making it difficult to find a threshold value for poor ovarian reserves without a significant overlap, with normal values.
Data from the literature have indicated that in PCOS, there is an excess of small follicles (2–5 mm) as compared to follicles of sizes, 6-9 mm among selected follicles. AMH has been considered to be a good marker of small follicles in both normal and PCOS women [8,12]. AMH is not under the influence of gonadotrophic hormones and it does not vary throughout the menstrual cycle and thus, it better reflects the number of follicular pool [11,14,15], the degree of maturation [16] and even the sensitivity to the action of FSH.
The increase in the concentrations of serum AMH in PCOS may be the result of excess of small follicles [17]. Increased production of AMH by granulosa cells [18] would be involved in the arrest of follicular development through negative action of FSH [19,12], thus further decreasing the sensitivity of follicles [20,16,21]. Thus, tonic increase of AMH may be involved in the arrest of follicular development, which is obviously more in PCOS.
The ROC AUC determines the sensitivity of the diagnostic test and it may vary between 0.5 (no discriminative power) and 1.0 (perfect discrimination). The AUC of serum AMH assay yielded a satisfying value of 0.851 in Pigny’s studies [12]. In his work, with a cut off value of 60 p mol/l, the serum AMH level showed a good specificity of 92%, but a relatively poor sensitivity of 67%. With a sensitivity of 75% and a specificity of 100% for a cut off value at 12, the follicle count appeared to be a better diagnostic tool than AMH measurement [22]. In our study, a perfect discrimination was observed between PCOS and controls and a specificity of 98% and a sensitivity of 93% were observed, with a cut off value of 3.34ng/ml. The discriminative power and sensitivity of AMH for PCOS was well established in our study and its higher level in serum reflected the severity of follicular arrest in PCOS of infertile group rather than novel measure of ovarian reserve.
Conclusion
The level of AMH was two times higher in PCOS, which did not mean that ovarian reserve was higher in PCOS, as AMH was a direct marker of ovarian reserve. The results of this study reflected that diagnostic potency of AUC for PCOS indicated that AMH was a potential marker of recruited non growing follicles rather than a simple marker of ovarian reserve.
[1]. Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER, Antral follicle counts by tranvaginal ultrsonography are related to age in women with proven natural fertilityFertil Steril 1999 72:845-51. [Google Scholar]
[2]. Gougeon A, Regulation of ovarian follicular development in primates: facts and hypothesesEndocr Rev 1996 17:3065-71. [Google Scholar]
[3]. Lee SJ, Lenton EA, Sexton L, Cooke ID, The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cyclesHum Reprod 1988 3:851-55. [Google Scholar]
[4]. Klinkert ER, Broekmans FJ, Looman CW, Habbema JD, Te Velde ER, The antral follicle count is a better marker than basal follicle stimulating hormone for the selection of older patients with acceptable pregnancy prospects after in vitro fertilizationFertil Steril 2005b 83:811-14. [Google Scholar]
[5]. Muttukrishna S, MeGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P, Antral follicle count, anti-Mullerian hormone and inhibin: predictors of ovarin response in assisted reproductive technologyBJOG 2005 112:1384-90. [Google Scholar]
[6]. Te Velde Er, Pearson Pl, The variability of female reproductive ageingHum Reprod Update 2002 8:141-54. [Google Scholar]
[7]. Themmen APN, Anti-Mullerian hormone: its role in follicular growth intiation and survival and as an ovarian reserve markerJ Natl Cancer Inst 2005 34:18-21. [Google Scholar]
[8]. Laven JSE, Mulders AGMGJ, Visser JA, Themmen AP, De Jong FH, Fauser BCJM, AMH serum concentration in normoovulatory ana anovulatory women of reproductive ageJ Clin Endocrinol Metab 2004 89:318-23. [Google Scholar]
[9]. Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC, Changes in anti–Mullerian hormone serum concentration over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertilityHum Reprod 2004 19:2036-42. [Google Scholar]
[10]. Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapaninen JS, Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndromeHum Reprod 2005 20:1820-26. [Google Scholar]
[11]. Fanchin R, Schonauer IM, Righini C, Frydman N, Frydma R, Taieb J, Serum anti-Mullerian hormone during controlled ovarian hyperstimulationHum Reprod 2003 18:328-32. [Google Scholar]
[12]. Pigny P, Merlen E, Robert Y, Cortet-Rudelly C, Decanter C, Jonard S, Dewailly D, Elevated serum level of AHM in patients with PCOS, relation to the ovarian follicle excess and to the follicular arrestJ Clin Endocr Metab 2003 88:5957-62. [Google Scholar]
[13]. Jain T, Soules MR, Collins JA, Comparison of Basal follicle stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screeningFertil Steril 2004 82:180-85. [Google Scholar]
[14]. Viser JA, Themmen AP, Anti–Mullerian hormone and folliculogenesisMol Cell Endocrinol 2005 234:81-86. [Google Scholar]
[15]. Van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Serum anti–Mullerian hormone levels: a novel measure of ovarian reserveHum Reprod 2002 17:3065-71. [Google Scholar]
[16]. Andersen CY, Byskov AG, Estradiol and regulation of anti mullerian hormone, inhibin-A and inhibin- B secretion: analysis of small antral and preovulatory human follicles fluidJ Clin Endocrinol Metab 2006 91:4064-69. [Google Scholar]
[17]. Virser JA, de Jong FH, Laven JS, Themmen APN, Anti-Mullerian hormone: a new marker or ovarian functionReproduction 2006 131:1-9. [Google Scholar]
[18]. Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Granulosa: cell production of anti–Mullerian hormone is increased in polycystic ovariesJ Clin Endocrinol Metab 2007 92:240-45. [Google Scholar]
[19]. Jonard S, Dewailly D, The follicular excess in polycystic ovaries due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrestHum Reprod Update 2004 10:107-17. [Google Scholar]
[20]. Durlinger AL, Grujters MJ, Kramer P, Karels B, Matzuk MM, Rose UM, Anti-Mullerian hormone attenuates the effects of FSH on follicle development to the mouse ovaryEndocrinology 2001 142:4891-99. [Google Scholar]
[21]. Weenen C, Laven JSE, von Bergh ARM, Cranfield M, Groome NP, Visser JA, AMH expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitmentMol Hum Reprod 2004 10:77-83. [Google Scholar]
[22]. Jonard S, Robert Y, Corrtet Rudelli C, Pigny P, Decanter C, Dewailly D, Ultrasound examination of polycystic ovaries: is it worth counting the follicles?Hum Reprod 2003 18:598-603. [Google Scholar]