The metabolic syndrome characterized by insulin resistance, dyslipidemia, and hypertension is associated with increased risk of type 2 diabetes and cardiovascular disease, resulting in reduced quality of life and increased risk of mortality and morbidity. The prevalence of metabolic syndrome has dramatically increased worldwide due to a modern lifestyle and an increase in consumption of refined sugar particularly fructose [1]. It was demonstrated that in rats fed with high-fructose diet, the concentration of free radicals was three times higher than in the control group. Moreover, fructose addition brought about lowering of antioxidant levels. The human consumption of fructose has been increasing over the years. Epidemiological studies revealed direct association between fructose intake and coronary heart diseases [2].
However, little is known about the potential effects of garlic on body weight regulation or blood glucose lowering in vivo animal models of diabetes. Furthermore, the beneficial effects of garlic on insulin resistance and antioxidant status remain unclear. Hence, the present study was conducted in order to find the therapeutic potential of garlic as oral hypoglycaemic and antioxidant agent. The effects produced by these treatments are compared with the standard drug, metformin.
Material and Methods
Chemicals
Thiobarbituric acid and reduced glutathione (GSH) were procured from Sigma Chemicals Co. (St. Louis, MO, USA). All other chemicals were of analytical grade and were obtained from standard commercial suppliers.
Preparation of garlic extract
Fresh garlic (Allium Sativum L.) was purchased from local market. The 100g of garlic bulbs suspended in distilled water was boiled for 20 minutes at 100°C. the cooled water was removed by filtration and lyophilized to obtain a powder form. The prepared extract as stored at 4ºC.
Animals
Male albino Wistar rats in the age of 5 months, weighs between 200 to 250 g were housed in polypropylene cages with stainless steel grill. The animals were maintained at standard conditions with a 12-/12-hour light/dark cycle. The animal procedures were approved by the institutional animal ethics committee (Jip/Micro/Jiaec/2012 date 29.3.2012).
Experimental design
A total of 30 rats were selected for the study and randomized equally (6 rats) to all the study groups. The sample size was determined by convenience subjected to the Institute Animal Ethic Committee approval.
Group 1: control rats, received standard rat chow.
Group 2: control + garlic extract (25mg/kg body weight/day/rat) in aqueous solution orally for 60 days.
Group 3: High Fructose Diet (HFD) rats were given 60% fructose mixed with standard rat chow.
Group 4: HFD + garlic extract (25 mg/kg body weight/day/rat) in aqueous solution orally for 60 days.
Group 5: HFD + metformin (50mg/kg body weight/day/rat) in aqueous solution orally for 60 days [6].
Body weight gain
Changes in body weight of rats in all groups were noted throughout the experimental period. The weight of each rat was recorded on day one of the study and at weekly intervals throughout the study.
Intraperitoneal glucose tolerance test (IPGTT)
At the end of the experimental period, IPGTT was performed in all groups of rats. The 12 hour fasted animals were challenged with a glucose at a dose of 2 g / kg body weight intraperitoneally. Blood samples were collected at 0 min (before glucose administration), 30, 60 and 120 min after glucose administration [7].
Biochemical analyses
At the end of the experiment, the plasma glucose was estimated using the glucose oxidase-peroxidase method in a fully automated clinical chemistry analyzer (Olymbus AU-400) and the remaining samples were stored at -80°C for estimation of TNF-alpha (Diaclone, France) and insulin (Crystal chem. inc, USA) by ELISA method according to the manufacturer’s instructions. The Homeostasis Model Insulin Resistance (HOMA-IR) was calculated using the formula [fasting glucose (mmol/L) X fasting insulin (µIU/mL)]/22.5. The plasma malondialdehyde were estimated by TBARS method [8,9]. The whole blood reduced glutathione (GSH) was measured using Ellman’s reagent (5,5’-Dithio-bis-2-nitrobenzoic acid,) as described by Beutle et al., [10]. Haemoglobin content of blood was estimated by cyanmethemoglobin method with Drabkin reagent and catalase assay the method of Aebi. The Total Antioxidant Status (TAS) was measured by FRAP method [11–13].
Statistical Analysis
All values were expressed as mean ± S.D. Differences between the groups were assessed using one way analysis of variance with (ANOVA) with Tukey post hoc test. All statistical analysis was carried out using SPSS version 19.0.
Results
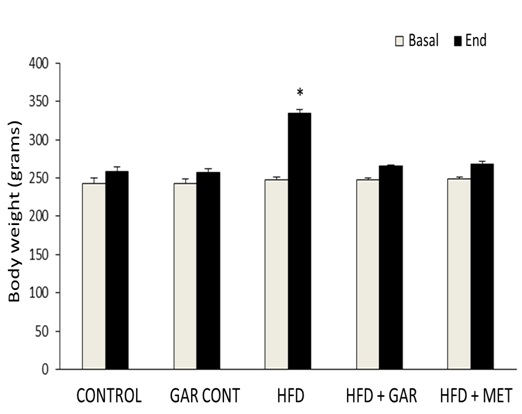
[Table/Fig-1] illustrates the effect of body weight on the control and experimental groups of rats. The HFD induced diabetic rats showed a significant (p < 0.05) increase in the body weight. A significant (p < 0.05) decline in the body weight was observed on oral administration of garlic as well as metformin.
Illustrates the effect of body weight of the control and experimental groups of rats. (Significantly different with control, bSignificantly different with garlic group, csignificantlydifferent with HFD+metformin at 5% level of significant (p<0.05)
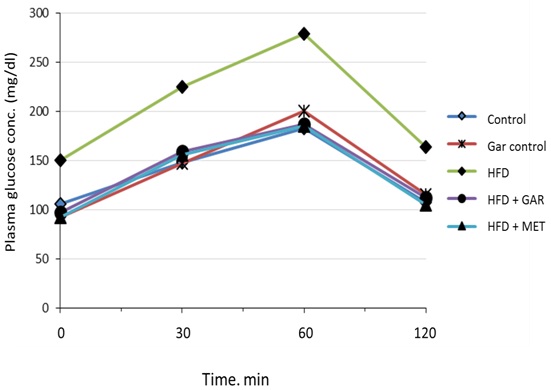
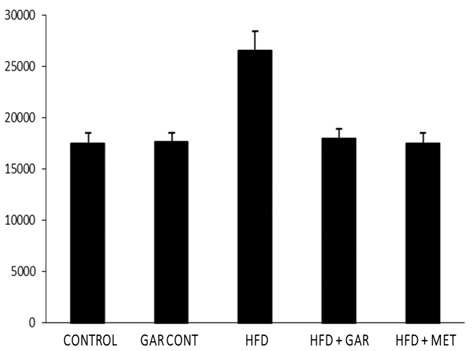
[Table/Fig-2] shows that, the effect of glucose tolerance test in male wistar rats. High fructose feeding showed impaired glucose tolerance and an increase in AUC. The analysis of the IPGTT at the end of the feeding period and the comparison between area under the curve (AUC) between control and experimental groups showed that fructose-fed rats developed glucose intolerance [Table/Fig-3]. Treatment with garlic as well as metformin significantly improved the glucose intolerance caused by high fructose.
Effect of garlic administration on intraperitoneal glucose tolerance test. (Significantly different with control, bSignificantly different with garlic group, csignificantlydifferent with HFD+metformin at 5% level of significant (p<0.05)
Incremental area under the curve (AUC) of IPGTT. (Area under the curve (AUC) of different groups was calculated using NCCS software. Significantly different with control, bSignificantly different with garlic group, csignificantly different with HFD+metformin at 5% level of significant (p<0.05))
[Table/Fig-4] depicts the effect of garlic on the plasma glucose, insulin and TNF-alpha in control and experimental groups of rats. The plasma glucose, insulin, IR and TNF-alpha levels in HFD-induced diabetic group of rats were significantly (p < 0.05) increased, when compared with control group of rats. The oral treatment with garlic as well as metformin significantly (p < 0.05) decreased the glucose, insulin and TNF-alpha when compared with HFD group of rats.
Comparison of the levels of plasma glucose, insulin, HOMA-IR and TNF–α between the groups.
| Groups | Fasting plasma Glucose (mmol/L) | Plasma insulin (µIU/ml) | HOMA-IR | TNF – α (pg/ml) |
|---|
| Control | 6.0 ± 0.51 | 31.25 ± 13.86 | 8.32 ± 3.86 | 6.52 ± 3.28 |
| Control+ garlic | 5.2 ± 0.41 | 22.59 ± 9.61 | 5.16 ± 1.85 | 5.49 ± 2.50 |
| HFD | 8.2 ± 0.80b,c | 105.90 ± 20.80 a,b,c | 38.7 ± 5.76 a,b,c | 102.73 ± 28.55 a,b,c |
| HFD+ garlic | 5.4 ± 0.44 | 60.97 ± 26.32 | 14.5 ± 6.41 | 8.60 ± 3.99 |
| HFD+ metformin | 5.2± 0.81 | 56.38 ± 12.04 | 12.9 ± 3.27 | 4.47 ± 0.99 |
| p Value | p<0.05 | p<0.05 | p<0.05 | p<0.05 |
(aSignificantly different with control, bSignificantly different with garlic group, csignificantly different with HFD+metformin at 5% level of significant (p<0.05).
[Table/Fig-5] indicates the effect of garlic on the malondialdehyde level and antioxidants enzymes in the control and experimental groups of rats. In high fructose rats, there was a significant elevation in the level of malondialdehyde and a concomitant decrease in the levels of catalase, GPx, GSH and TAS when compared with control rats. Oral treatment with garlic brought back them to near normal levels as was the case with metformin treatment. There was no statistical significant difference in these parameters between garlic treated and the control rats. This illustrates the complete normalization of oxidant status with garlic treatment.
Antioxidants effects of garlic in control and experimental groups of rats.
| Groups | MDA(µM/L) | CAT Katal /ml | GPx(mg/gHb) | GSH mg/g Hb | TAOS (µM/L) |
|---|
| Control | 2.62 ± 0.41 | 44.04 ± 16.9 | 43.3 ± 7.2 | 6.74 ± 1.4 | 1066.7 ± 82.41 |
| Control+ garlic | 2.75 ± 0.24 | 49.12 ± 19.6 | 53.15 ± 13.1 | 9.87 ± 1.4 | 1149.7 ± 69.97 |
| HFD | 4.23 ± 0.87 a,b,c | 30.99 ± 4.64a,b,c | 29.89 ± 5.0a,b,c | 3.59 ± 0.6a,b,c | 272.4 ± 22.08a,b,c |
| HFD+ garlic | 2.60 ± 0.30 | 51.18 ± 12.7 | 50.14 ± 2.2 | 6.69 ± 3.4 | 697.8 ± 54.19 |
| HFD+ metformin | 2.53 ± 0.39 | 57.54 ± 16.5 | 52.13 ± 6.0 | 7.40 ± 1.2 | 687.2 ± 51.9 |
| p Value | p<0.05 | p>0.05 | p<0.05 | p<0.05 | p<0.05 |
aSignificantly different with control, bSignificantly different with garlic group, csignificantly different with HFD+metformin at 5% level of significant (p<0.05)
Discussion
Dietary fructose is a monosaccharide which can induce metabolic syndrome which is of pathophysiologic importance for the development of diabetes and atherosclerosis. There are many reports in the literature describing an increase in body weight, hyperglycemia, and insulinemia with the consumption of high fructose diets in both humans and animal models [14–15]. Our results are consistent with earlier studies which found that the consumption of high fructose diets markedly induce hyperglycemia associated with hyperinsulinemia.
The significant increase in AUCs of glucose level after glucose loading during OGTT are seen in rats fed with high fructose diet, indicating that the ability of insulin to stimulate glucose disposal is markedly impaired in tissues by fructose feeding. As per the emerging evidence that prolonged consumption of high fructose diets contributes to excessive formation of Reactive Oxygen Species (ROS). This leads to oxidative stress and insulin resistance [16]. Moreover, an increase in cellular ROS directly triggers the activation of serine/threonine kinase cascades such as c-Jun N-terminal kinase and nuclear factor-kappa B that in turn phosphorylate multiple targets including the insulin receptor and the Insulin Receptor Substrate (IRS) [17]. Increased serine phosphorylation of IRS directly decreases its ability to undergo tyrosine phosphorylation and accelerates the degradation of IRS-1, causing impaired glucose uptake in tissues [18]. Our findings on the significantly salutary effect of garlic on glucose tolerance test are particularly promising and requires further elucidation. The blood glucose level regulated by garlic compounds (S-methylcysteine, S-allylcysteine and S-allylmercaptocysteine), may possibly contributed to this effect [19]. Reduction of body weight gain could also be responsible for improving insulin sensitivity in fructose fed rats.
High-fructose diet contributes to excessive formation of reactive oxygen species. This leads to oxidative stress and its associated complications like chronic inflammation, characterized by abnormal cytokine production (TNF-α) and the activation of a cascade of inflammatory signaling pathways [20]. TNF-α has been shown to enhance adipocyte lipolysis, which further increases free fatty acids and also elicits its own direct negative effects on the insulin signaling pathway by altering the tyrosine/serine phosphorylation of Insulin Receptor Substrate (IRS). Therefore, it is presumed that the reduction of pro-inflammatory mediators by garlic treatment might have facilitated/improved IRS-phosphorylation including metabolic syndrome. Such improved cellular events be able to reverse the inhibitory effect on insulin signalling pathways, mainly the enzymes of fatty acids, glucose uptake process and depletion of free fatty acids synthesis.
Increasing evidence in both experimental and human studies indicates that oxidative stress plays a major role in the pathogenesis of Type 2 diabetes. Free radicals are generated in diabetes by glucose oxidation. High levels of free radicals and the simultaneous decline of exogenous and endogenous antioxidants can lead to damage of cellular organelles and development of insulin resistance and decreased antioxidant enzymes [21]. In the present study, high fructose feeding increased oxidative stress. The significant restoration of redox status by treatment of garlic indicates its antioxidant property of garlic and may be administered as an adjuvant in the treatment of diabetes.
Conclusion
In conclusion, the supplementation of garlic to rats fed a high fructose diet prevents the development of oxidative stress and its associated complications include hyperglycemia and hyperinsulinemia. Further human studies are essential to explore the molecular mechanism of garlic in controlling the insulin resistance and its metabolic complications.
(aSignificantly different with control, bSignificantly different with garlic group, csignificantly different with HFD+metformin at 5% level of significant (p<0.05).
aSignificantly different with control, bSignificantly different with garlic group, csignificantly different with HFD+metformin at 5% level of significant (p<0.05)