Osteoarthritis (OA) is a common degenerative joint disease which leads to pain, stiffness, a reduced motion, swelling, crepitus, and disability. The knee is the most clinically significant site of primary osteoarthritis involvement. It is characterised by the progressive destruction of articular cartilage with joint-space narrowing, osteophyte formation, subchondral bone sclerosis and synovitis. Osteoarthritis continues to impose an enormous health and economic burden on society, especially among the elderly [1]. The aetiology and pathogenesis of OA remain poorly understood, but they have been associated with several risk factors such as aging, obesity, and traumatic injuries [2]. Nevertheless, biochemical factors have by now been recognised as playing an important role in OA development.
Increasing evidences in both experimental and clinical studies suggest that oxidative stress is considered to be one of the main causative factors in the pathogenesis of OA [3–7]. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are produced under physiological conditions in the human body and they are removed by cellular antioxidant defense system. The primarily formed radical is normally, superoxide radical (O2–); however, it may be converted to more harmful species such as hydroxyl radicals (OH–) and hydrogen peroxide (H2O2). These reactive oxygen species can result in oxidative damage to various components of the joint, which include collagen, proteoglycans and hyaluronan [6]. In general, lipid peroxidation was increased because of the diminished anti-oxidant defense mechanisms. The end-product of this damage is malondialdehyde (MDA). Elevated levels of MDA have been reported in plasma and synovial fluid of OA patients [3]. Nitrite (NO2–) is decomposition product of nitric oxide and it is a stable metabolite. Nitrite can be used as a biochemical marker for indirectly determining the presence of nitric oxide and its presence has been reported in plasma and synovial fluid of patients with OA [7]. Inducible nitric oxide synthase (iNOS) catalyzes the formation of intra-articular nitric oxide after its exposure to proinflammatory cytokines [8]. Enzymatic antioxidant system includes superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT), whereas and non-enzymatic antioxidant system contains glutathione (GSH), ascorbic acid (vitamin C), alpha-tocopherol (vitamin E), and carotinoids. These antioxidant defences exist both intra- and extracellulary, to protect tissue damage which results from oxidative stress [9]. In addition, the Ferric Reducing Antioxidant Power (FRAP) and Trolox Equivalent Antioxidant Capacity (TEAC) assay were utilized to measure the total antioxidant status, which has been assigned as Total Antioxidant Capacity (TAC). Increased MDA and decreased glutathione (GSH), ascorbic acid, vitamin E, and catalase activities were evident in OA patients [4]. A recent study also showed that synovial fluid vitamin E levels were significantly lower in the OA patients than in the patients with injured knee joints, which suggested the role of oxidative stress in OA [5].
We hypothesized that oxidative stress would increase, whereas antioxidant status would decrease in knee osteoarthritis. Therefore, the purpose of this study was to evaluate the oxidative stress and antioxidant statuses in plasma and synovial fluid in patients with primary knee osteoarthritis.
Material and Methods
Study Subjects
The present study was conducted in agreement with the guidelines of the Declaration of Helsinki. Written informed consents were obtained from the patients and healthy volunteers prior to their participation in the study. This study was approved by the Institutional Review Board on Human Research of the Faculty of Medicine, Chulalongkorn University. The sample size was designed according to the standardized effect size of 0.7, by using Student’s t-test to compare means of continuous variables, with a statistical power of 0.8, and a p-value of 0.05. Therefore, 35 patients were required in each group.
Thirty five OA patients (24 females and 11 males with a mean age of 69.2 ± 1.3 years and a mean body mass index (BMI) of 26.6 ± 0.6 kg/m2) who were diagnosed with clinical and radiographic evidences of knee osteoarthritis according to the criteria of the American College of Rheumatology were enrolled in the present study. OA patients were defined as having radiographic knee OA of Kellgren and Lawrence grade 3-4 (moderate to severe osteoarthritis) in at least one knee. The grading of the worst affected knee in each patient was used for data analysis. We also recruited 35 gender and age matched subjects (26 females and 9 males with a mean age of 68.6 ± 1.2 years and a mean BMI of 23.5 ± 0.5 kg/m2) with normal knee radiographs as controls. None of the participants had underlying diseases such as diabetes, a history of corticosteroid medication, other forms of arthritis, cancer, or other chronic inflammatory diseases.
Laboratory Methods
Venous blood samples which were taken from the cubital veins of each participant were centrifuged and they were stored immediately at -80°C until they were analyzed. Synovial fluid was collected from OA patients who were undergoing total knee replacement. The specimens were then instantly centrifuged to remove cells and joint debris and they were stored at -80°C until they were used for making measurements.
Determination of Nitrite
Nitrite levels in plasma and synovial fluid were measured by the Griess reaction (Promega, Madison, USA) by using sulfanilamide and N-1-napthylenediamine dihydrochloride (NED) under acidic (phosphoric acid) conditions. Formation of the azo compound was determined via its absorbance at 540 nm.
Determination of Malondialdehyde
Malondialdehyde (MDA) in plasma and synovial fluid was determined by its reaction with 2-thiobarbituric acid (TBA) (Sigma-Aldrich, St. Louis, USA) at 95°C [10]. MDA and TBA react together to produce a pink chromogen which has an absorbance at 532 nm. MDA (Sigma-Aldrich, St. Louis, USA) was used as the standard.
Determination of vitamin E by reverse phase-High Performance Liquid Chromatography (RP-HPLC)
Plasma or synovial fluid samples were extracted with ethanol (Merck, Germany) and hexane (Merck, Germany). Alpha-tocopheryl acetate (Sigma-Aldrich, St. Louis, USA) was used as the internal standard. After drying the hexane layer under nitrogen gas and resuspending it in methanol (Merck, Germany), the extract was injected into the HPLC system. The stationary phase was established by using a C18 column (Inertsil ODS-3, GL Sciences, Japan). The mobile phase was a mixture of isocratic methanol/water (98/2, v/v) at a flow rate of 1.5 ml/min. The HPLC peaks were detected by a UV detector at 292 nm [11].
Determination of total antioxidant capacity by trolox equivalent antioxidant capacity assay
The Trolox Equivalent Antioxidant Capacity (TEAC) assay is based on the scavenging of the 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma-Aldrich, St. Louis, USA) radical anion (ABTS•) convert into a colourless product [12]. The decrease in absorption at 734 nm after 6 minutes of addition of a test compound was used for calculating TEAC values. Different concentrations of Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) (Sigma-Aldrich, St. Louis, USA) were used to create a calibration curve.
Determination of total antioxidant capacity by ferric reducing antioxidant power
Total antioxidant capacity was measured by FRAP assay, as has been described by Benzie and Strain [13]. This assay depends upon the reduction of ferric tripyridyltriazine (Fe3+-TPTZ) (Sigma-Aldrich, St. Louis, USA) complex to ferrous tripyridyltrainzine (Fe2+-TPTZ) form at low pH and the absorbance was then determined at 593 nm. Ferrous sulfate (FeSO4) (Sigma-Aldrich, St. Louis, USA) was used as the standard.
Statistical Analysis
Statistical analysis was carried out by using SPSS software, version 19.0 for Windows (SPSS Inc., Chicago, USA). The data were expressed as means ± standard error of the mean (SEM). Comparisons of demographic data between OA patients and healthy controls were analyzed by using Chi-square and Student’s unpaired t-test. Differences between groups were assessed by using one way analysis of variance (ANOVA) with LSD post hoc test if ANOVA showed significance. Correlation between numerical data was acquired by using Pearson’s correlation coefficient (r). p-values of < 0.05 were considered to be statistically significant.
Results
Baseline Characteristics
There were no significant differences in age, gender, and body mass index (BMI) between knee OA patients and healthy controls. The baseline clinical parameters of the two groups have been demonstrated in [Table/Fig-1].
The baseline characteristics between knee OA patients and healthy controls
| Characteristics | Knee OA Patients (n=35) | Healthy Controls (n=35) | p |
|---|
| Age (years) | 69.2 ± 1.3 | 68.6 ± 1.2 | 0.4 |
| Gender (male/female) | 11/24 | 9/26 | 0.5 |
| BMI (kg/m2) | 26.6 ± 0.6 | 23.5 ± 0.5 | 0.2 |
Oxidative Stress, Vitamin E, and Antioxidant Capacity in Plasma and SF
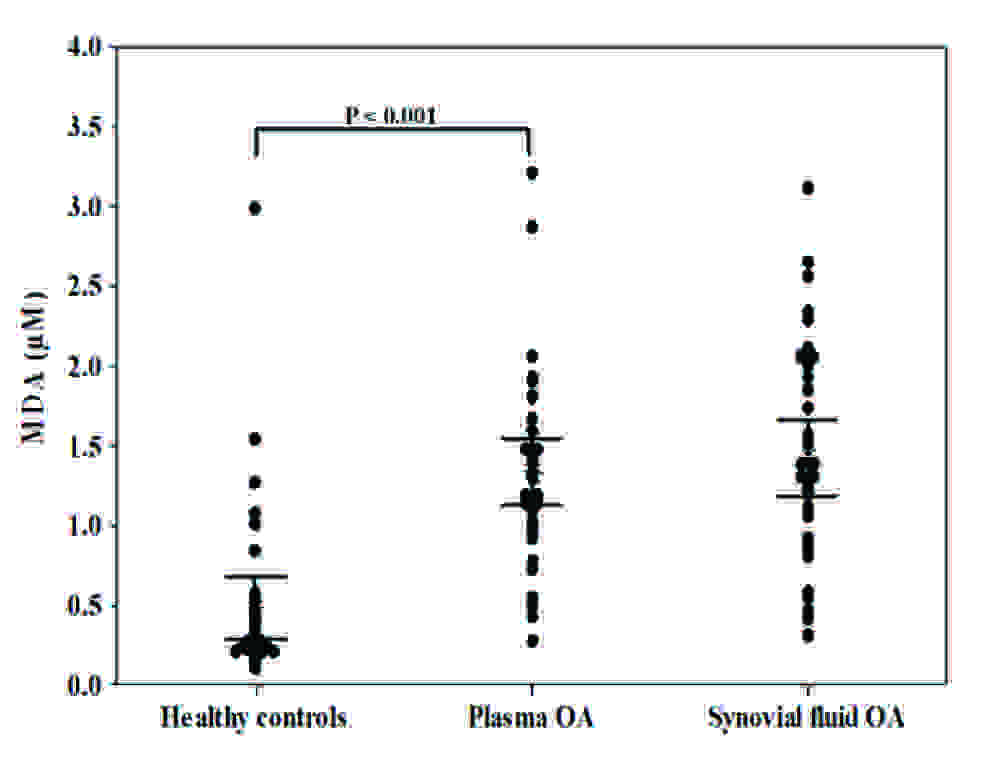
The plasma and synovial fluid concentrations of nitrite have been presented in [Table/Fig-2A]. OA patients had significantly higher plasma nitrite concentrations as compared to healthy controls (5.6 ± 0.6 μM vs. 3.4 ± 0.3 μM, p = 0.037). Nitrite levels in synovial fluid were higher than those in paired plasma samples but there was not statistical significance (6.0 ± 1.1 μM vs. 5.6 ± 0.6 μM, p = 0.8). There was no correlation between plasma and synovial fluid nitrite concentrations in OA patients (r = 0.27, p = 0.12). Furthermore, MDA plasma concentrations were significantly higher in OA patients than in healthy controls (1.3 ± 0.1 μM vs. 0.5 ± 0.1 μM, p < 0.001), as has been shown in [Table/Fig-2B]. Moreover, MDA levels in synovial fluid were higher than those in paired plasma samples but there was no significant difference (1.4 ± 0.1 μM vs. 1.3 ± 0.1 μM, p = 0.6). There was no correlation between plasma and synovial fluid MDA levels in OA patients (r = 0.26, p = 0.13).
Nitrite levels in plasma and synovial fluid of patients with OA and healthy controls.
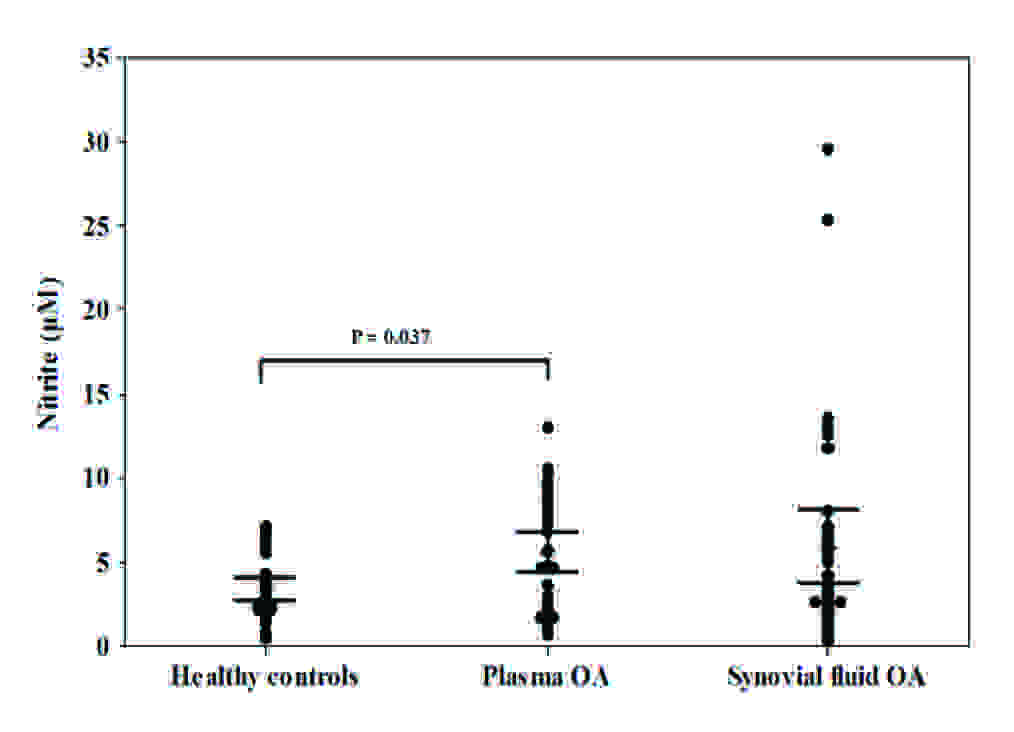
MDA levels in plasma and synovial fluid of patients with OA and healthy controls.
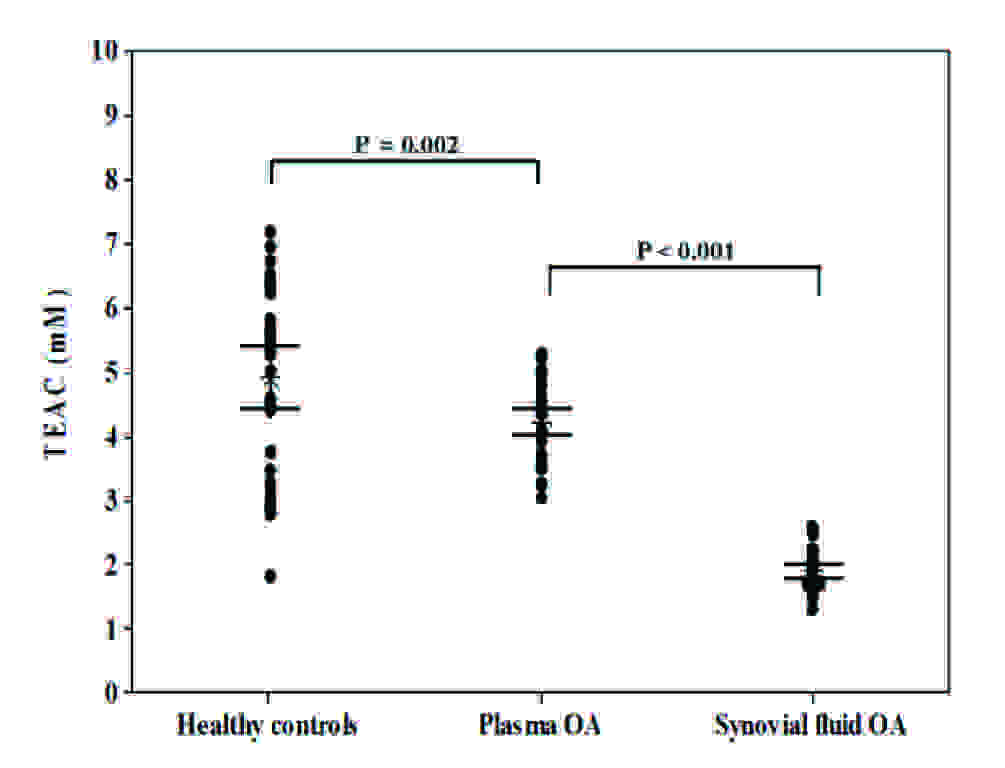
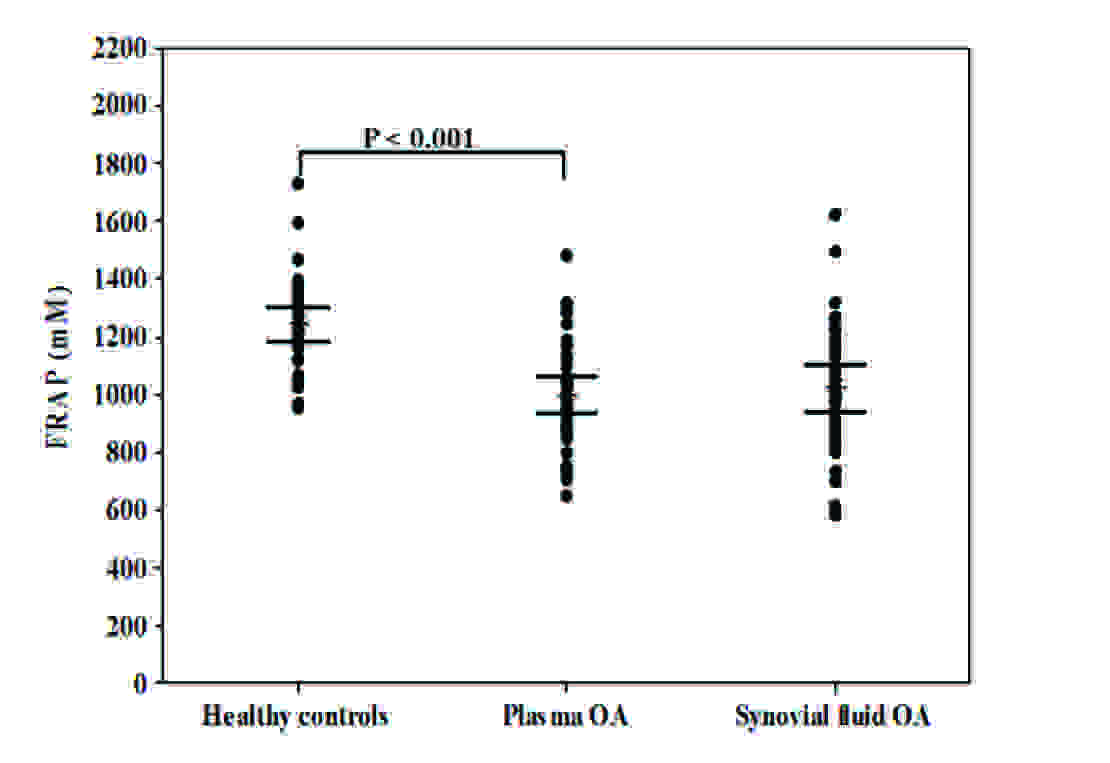
Subsequently, plasma vitamin E levels were found to be significantly lower in OA patients than in healthy controls (16.0 ± 1.1 μg/ml vs. 20.2 ± 0.8 μg/ml, p < 0.001) [Table/Fig-2C]. Vitamin E levels in synovial fluid of OA patients were significantly lower than those in paired plasma samples (4.5 ± 0.3 μg/ml vs. 16.0 ± 1.1 μg/ml, p < 0.001) [Table/Fig-2C]. Moreover, the relationship between the plasma and synovial fluid vitamin E concentrations in OA patients was examined. No correlation was evident between the plasma and synovial fluid vitamin E levels (r = 0.27, p = 0.12). The total antioxidant capacity, as was measured by TEAC assay in plasma of OA patients, was significantly lower th an that in healthy controls (4.2 ± 0.1 mM vs. 5.0 ± 0.2 mM, p = 0.002). Further analysis revealed that TEAC levels in synovial fluid of OA patients were significantly lower than paired plasma samples (1.9 ± 0.1 mM vs. 4.2 ± 0.1 mM, p < 0.001), as have been displayed in [Table/Fig-2D]. No correlation was observed between the plasma and synovial fluid TEAC levels in OA patients (r = -0.25, p = 0.14). The mean concentration of plasma FRAP in OA patients was significantly lower than that in the healthy controls (998.2 ± 32.3 mM vs. 1242.8 ± 30.3 mM, p < 0.001) [Table/Fig-2E]. Although synovial fluid FRAP levels in OA patients were higher than paired plasma samples, the difference was not statistically significant (1022.5 ± 39.3 mM vs. 998.2 ± 32.3 mM, p = 0.6). There was a positive correlation between the plasma and synovial fluid FRAP levels in OA patients (r = 0.42, p = 0.01).
Vitamin E levels in plasma and synovial fluid of patients with OA and healthy controls.
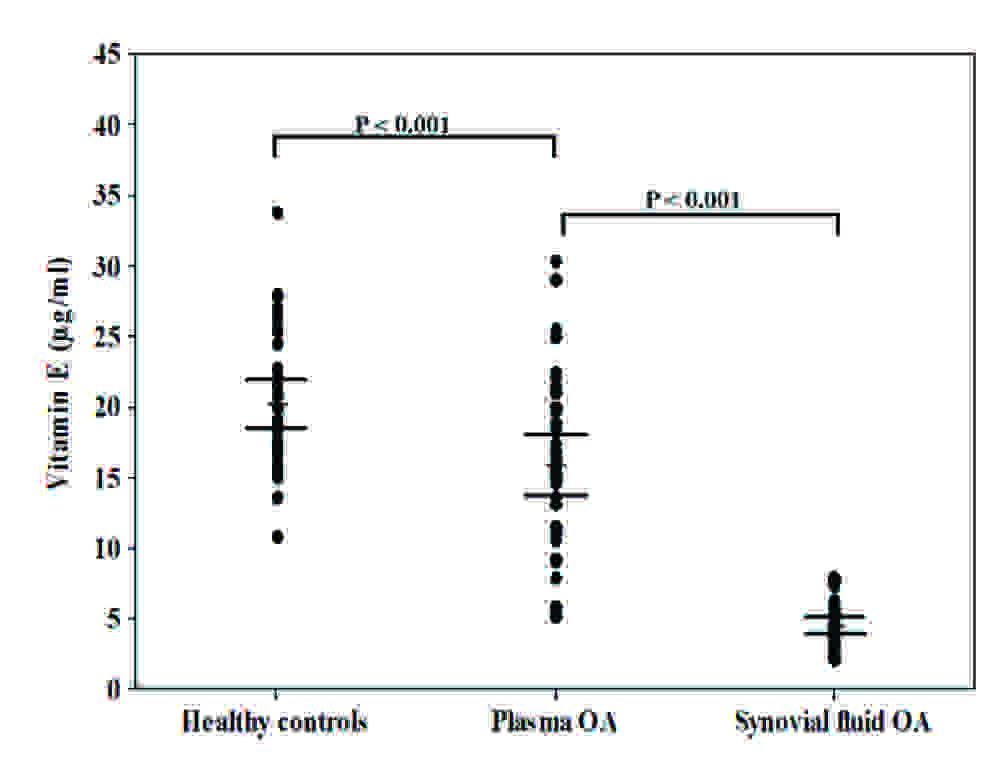
TEAC levels in plasma and synovial fluid of patients with OA and healthy controls.
FRAP levels in plasma and synovial fluid of patients with OA and healthy controls.
Association of Oxidative Stress, Vitamin E, and Antioxidant Capacity in SF
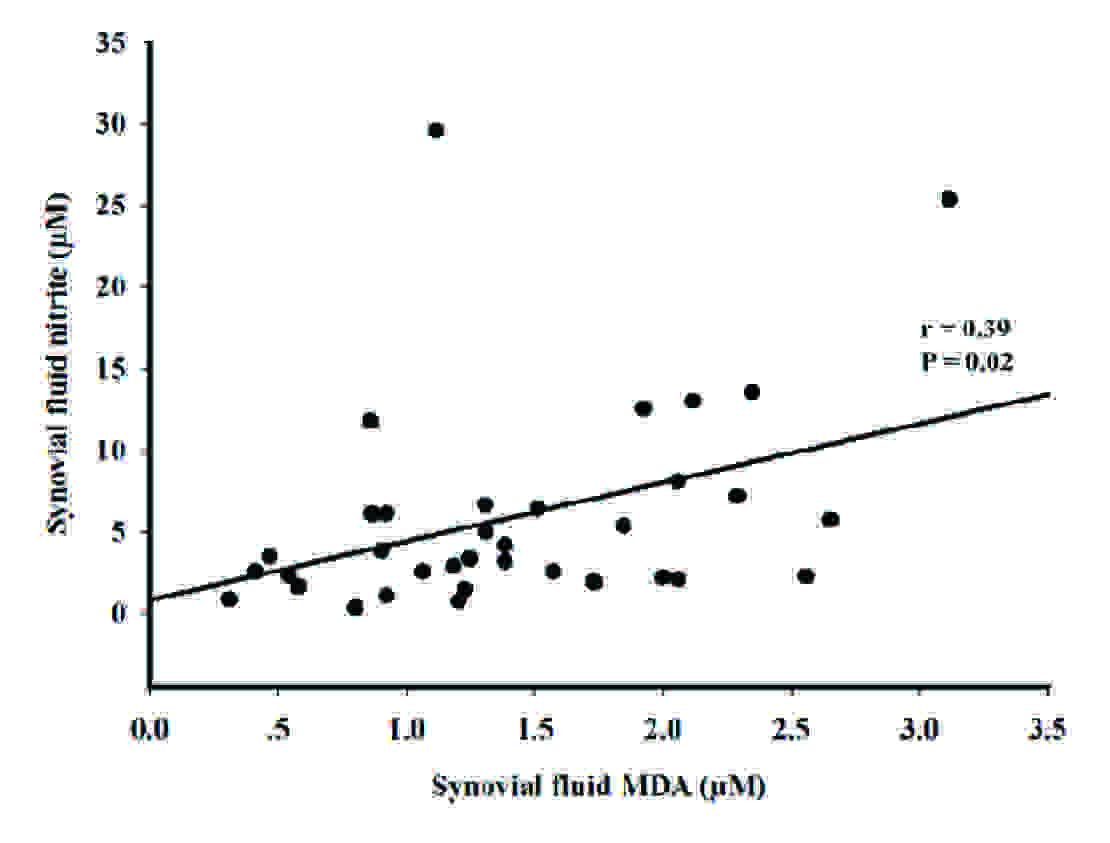
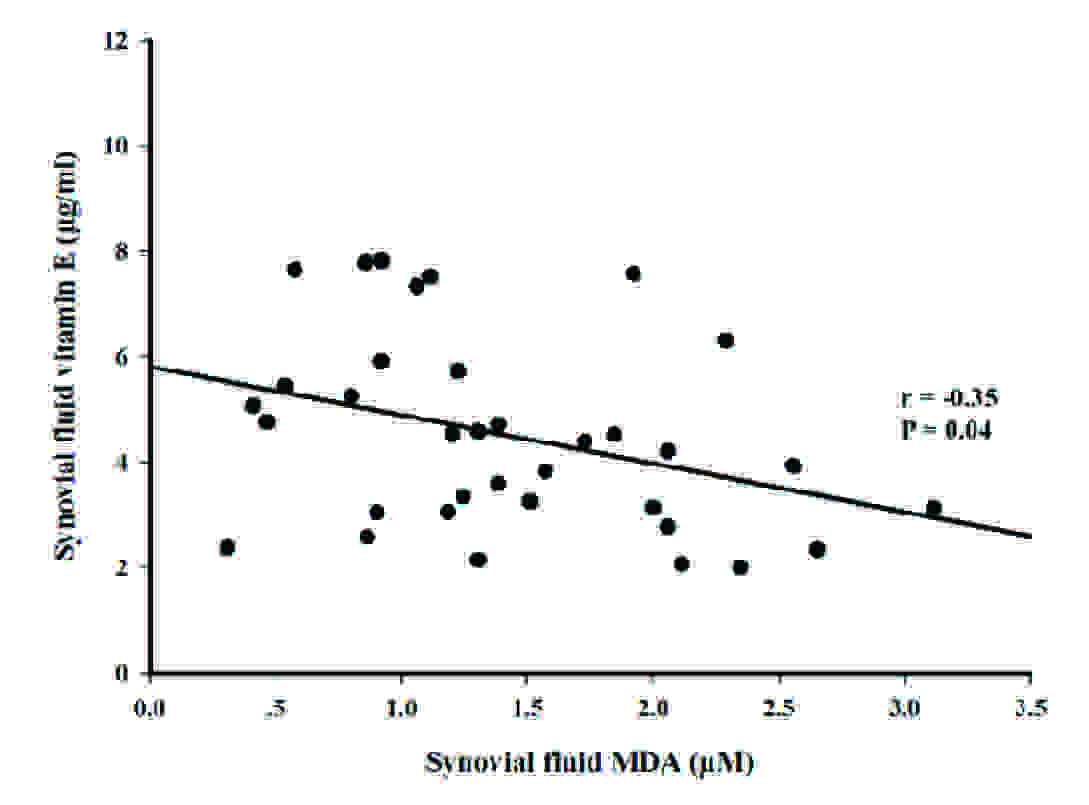
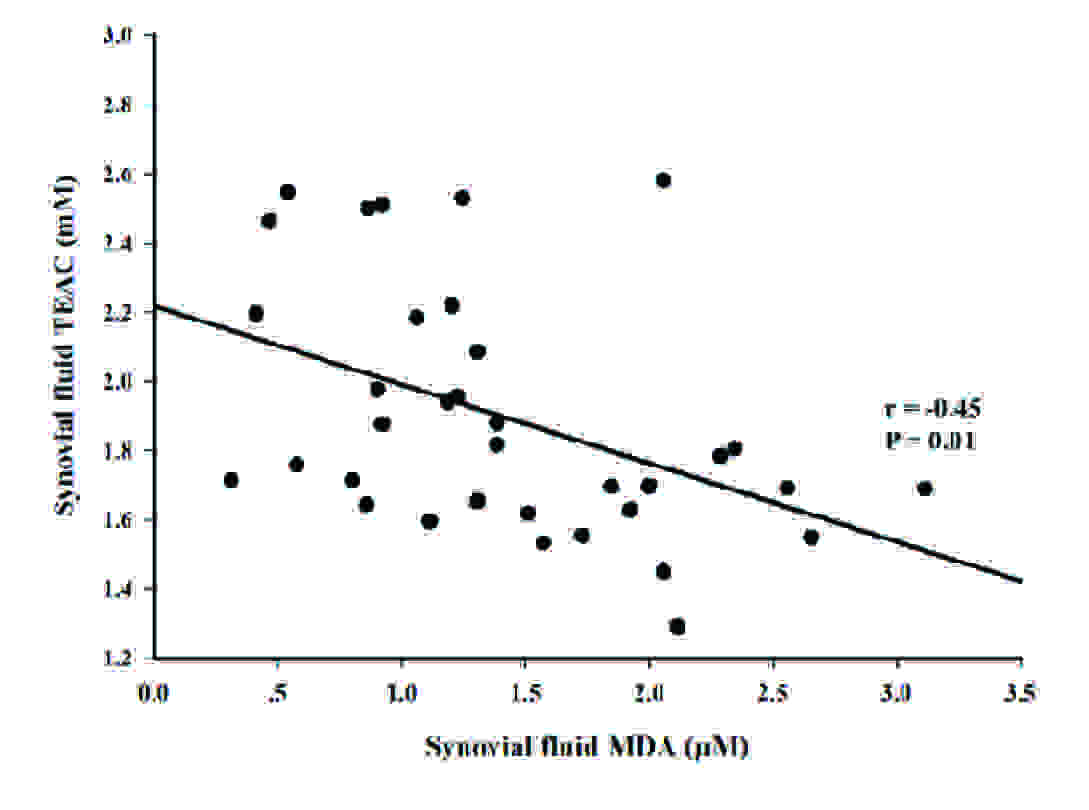
Subsequent analysis showed that synovial fluid MDA concentrations in OA patients were positively correlated with synovial fluid nitrite concentrations (r = 0.39, p = 0.02) [Table/Fig-3A]. In contrast, there was an inverse correlation between synovial fluid MDA concentrations in OA patients and synovial fluid vitamin E levels (r = -0.35, p = 0.04) [Table/Fig-3B] and synovial fluid TEAC levels (r = -0.45, p = 0.01) [Table/Fig-3C].
Correlation between the synovial fluid MDA levels and synovial fluid nitrite levels in OA
Correlation between the synovial fluid MDA levels and synovial fluid vitamin E levels in OA.
Correlation between the synovial fluid MDA levels and synovial fluid TEAC levels in OA.
Discussion
Although oxidative stress and antioxidant status have been investigated in osteoarthritis patients, there is no published data with regards to the correlation between oxidative stress and antioxidant status in primary knee OA. In this study, patients with primary knee OA had significantly higher plasma nitrite and MDA concentrations as compared to those in healthy controls, indicating that oxidative stress was involved in the pathogenesis of OA. Previous studies had reported that NO levels in synovial fluid were increased in OA patients [7, 8, 14, 15]. Likewise, MDA or lipid peroxidation was increased in plasma and synovial fluid of osteoarthritis and rheumatoid arthritis patients [3, 4, 16].
Vitamin E is one of the non-enzymatic antioxidant systems. Alpha-tocopherol is a major component of vitamin E that exhibits an antioxidant activity and which also plays a role in the prevention of cartilage degeneration [17]. In the current study, it appeared that vitamin E concentrations in plasma of OA patients were significantly lower than those in healthy controls. Moreover, synovial fluid vitamin E concentrations in OA patients were also significantly lower than paired plasma samples. In accordance with our findings, Sutipornpalangkul et al., revealed that synovial fluid vitamin E levels in OA patients were decreased as compared to those in patients with injured knee joints [5], suggesting an imbalance between oxidative stress and antioxidant capacity. Vitamin E may enhance chondrocyte growth, provide protection against ROS and RNS and exhibit an anti-inflammatory activity [17–19]. Moreover, several clinical studies which were done on vitamin E supplementation have shown the beneficial effect of vitamin E in OA patients [20, 21].
Our findings demonstrated an excessive degree of oxidative stress in OA patients, as was evidenced by an increase in oxidative stress parameters and a decrease in antioxidant parameters. In addition, TEAC levels in plasma and synovial fluid were significantly decreased in patients with OA. Furthermore, FRAP levels were also significantly lower in OA patients as compared to those in healthy controls. In contrast to our findings, Sarban and colleagues illustrated that plasma Total Antioxidant Capacity (TAC) levels were decreased in rheumatoid arthritis patients; however, there was no significant difference in the levels between OA patients and controls [3].
The explanation for these conflicting data with regards to antioxidant status is unclear, but it could be attributed to differences in the stage of disease, populations or assays which are applied, or incomplete control for confounding variables.
Regarding the pathogenesis of degenerative diseases which include diabetes mellitus [22, 23], cardiovascular disease [24], and OA [25, 26], oxidative stress is an important mechanism. An oxidant-antioxidant imbalance leads to pathophysiological effects which are associated with OA, such as joint inflammation, cartilage damage, and synovitis [27, 28]. The development of preventive and therapeutic approaches should be considered for OA, for decreasing oxidative stress and for increasing antioxidants in blood circulation and local tissues of OA patients.
It should be mentioned that this study had some limitations. This research was administered as a single-centre trial with a relatively small sample size. Prospective studies which are conducted on random samples from multiple centres, which have larger sample sizes, are necessary to validate our data. Additionally, synovial fluid samples from healthy controls were not taken due to ethical reasons. Further research on synovial samples from controls will be essential, for a better understanding of our findings. Finally, as this study was designed as a cross-sectional study; absolute cause and effect relationships may not be possible. Prospective longitudinal investigations are warranted for demonstrating disease progression and for defining the precise role of oxidative stress and antioxidant status in knee OA.
In conclusion, the present study illustrated that plasma oxidative stress parameters in OA patients were higher than those in healthy controls and that plasma antioxidant parameters in OA patients were lower than those in healthy controls. These findings indicated that oxidative stress could be one of the major causative factors in osteoarthritis. However, further studies will be needed to examine the effect of antioxidant supplementation on severity of OA.