Aortic Valve Annular Dimension in Indian Population
Hannah Sugirthabai Rajila Rajendran1, Sudha Seshayyan2, Ashok Victor3, Gangadevi Rajapandian4
1 Associate Professor, Department of Anatomy, Chettinad Hospital and Research Institute, Kelambakkam,Chennai, TamilNadu,India.
2 Director, Institute of Anatomy, Madras Medical College, Chennai, Tamil Nadu, India.
3 Assistant Professor, Department of Cardiology, Stanley Medical College, Chennai, TamilNadu,India-600001.
4 Assistant Professor, Department of Radiodiagnosis, Stanley Medical College, Chennai, TamilNadu, India-600001.
NAME, ADDRESS, E-MAIL ID OF THE CORESPONDING AUTHOR: Dr. Hannah Sugirthabai Rajila Rajendran, 7D, KG Towers, 30 & 30A, Bypass Road, Velachery, Chennai -600042
Phone: 9710403803,
E-mail: ashrajsanada@gmail.com
Aim: The Aortic Valve (AV) annular dimension with respect to the Body Surface Area (BSA) of the Indian population is compared against the standard values. Presence of discrepancies can lead to patient prosthesis mismatch during aortic valve replacement surgeries.
Methods: This study was conducted on 406 subjects. AV diameter was examined by using parasternal long axis view, where the imaging plane transects the AV in an anteroposterior direction and its x axis is aligned parallel to the long axis of aorta. Data were statistically analysed with western population.
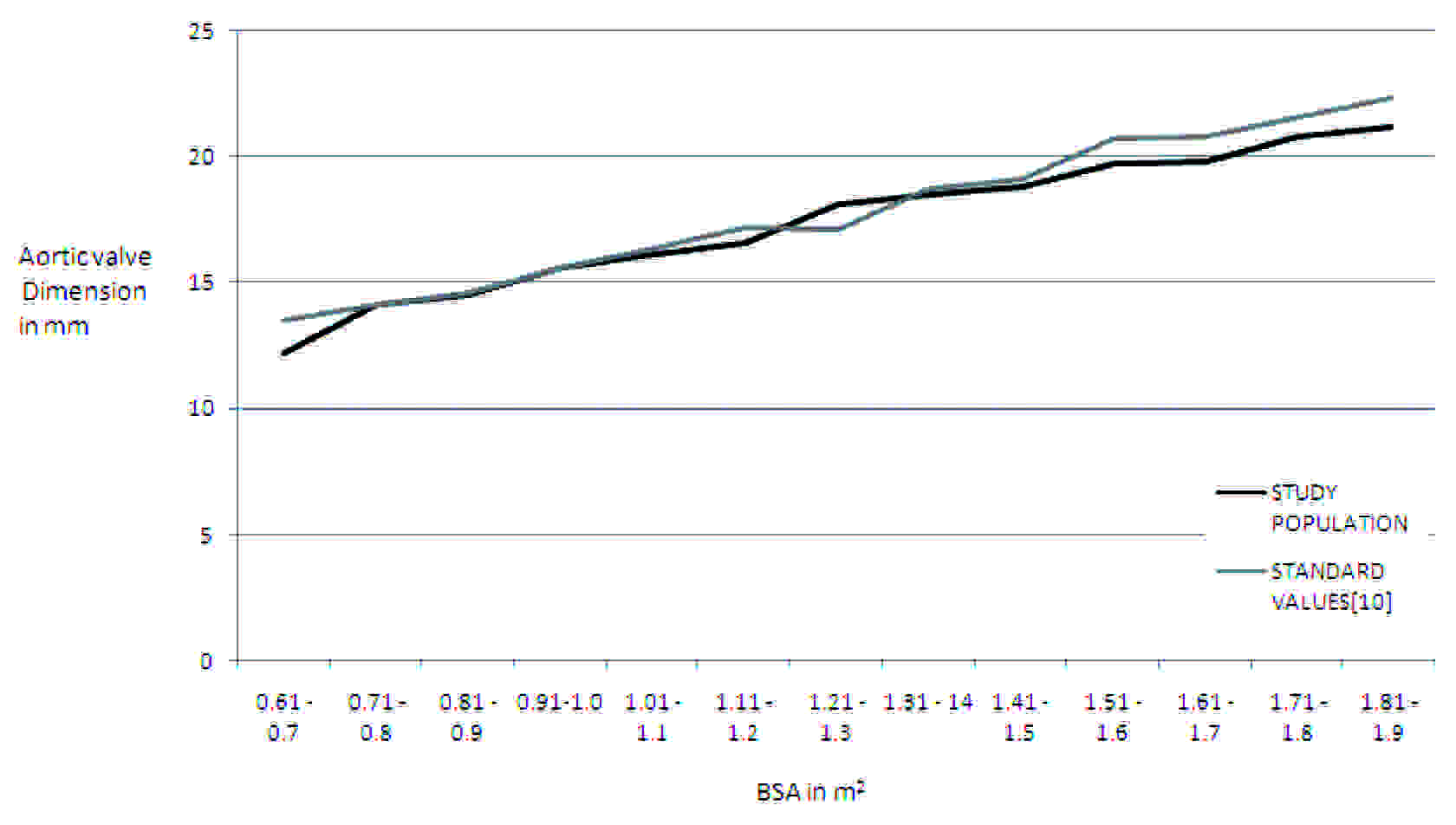
Results: The AV dimension ranged from 12.2 mm to 21.2 mm in the BSA range of 0.6 to 1.9 m2, showing a linear increase in diameter with increasing BSA. There was an increase of about 2 mm, from 0.61 - 0.7 m2 BSA to 0.71 - 0.8 m2 BSA. A linear increase which ranged from 0.3 to 1 mm was observed for BSA which ranged from 0.81 m2 to 1.2 m2. In the BSA range of 1.21 – 1.3 m2, there was an increase of 1.5 mm. A steady increase which ranged from 0.4–1 mm was observed in the BSA which ranged from 1.31- 1.9 m2.
Conclusions: There is a significant difference between Indian and western population in the aortic dimension, in the body surface ranges of 0.61-0.7, 1.11-1.2, 1.21-1.3, 1.51-1.6, 1.61-1.7, 1.71-1.8 and 1.8-1.9 m2. In the range of 1.21-1.3 m2, the diameter was larger than standard, whereas in all the other ranges, AV diameter was smaller than standard values. BSA, as a good predictor of AV dimension, has also been proved.
Aortic valve annulus, BSA, Indian population
Introduction
Cardiac valves are replaced in either stenotic or regurgitant lesions. To replace the valve and to know the severity of the regurgitant lesion, the normal valve annular size should be known. Patient prosthesis mismatch is a complication if the patient’s annulus is different from the size of the prosthetic valve. The term, ‘patient prosthesis mismatch’ was introduced by Rahimtoola [1], who stated that an obstructive aortic prosthetic valve with an effective orifice area of less than 0.8cm2/m2 could increase the operative mortality and impair functional recovery after an aortic valve replacement. Philippe Pibarot and Jean G Dumesnil [2] stated that a patient–prosthesis mismatch was associated with worse haemodynamics, a reduced regression of LV hypertrophy, more cardiac events and increased short term and long term mortalities after valve replacements.
Habbal ME, Somerville J [3], in 1989, showed that if the dimensions of cardiac valves were corrected with body surface area (BSA), the two dimensional echo and surgical measurements could be identical. Thus, BSA is a useful tool for estimating normal aortic valve size. Capps SB, Elkins RC and Frank DM [4] showed that in males, the aortic valve diameter was 23.1 ± 2mm and that in females, it was 21.0 ± 1.8 mm and that the pulmonary valve diameter was 26.2 ± 2.3mm in males and that it was 33.9 ± 2.2mm in females. The indexed aortic valve area was 2.02 ± 0.52cm2/m2 and pulmonary valve area was 2.65 ± 0.52cm2/m2. They showed that aortic valve and pulmonary valve diameter were closely related to body size.
When a valve is being replaced, a patient–prosthesis mismatch can result in restrictive changes of the heart, due to the prosthetic valve being smaller than the annular size of the valve. The annular dimensions depend on age, sex and individual’s BSA. (Capps SB, Elkins RC, Frank DM [4] and ZHU Dan and ZHAO Qiang [5]) These dimensions are of utmost significance for repair and replacement of cardiac valves. These parameters have been already studied in the western population and the standard measurements have been derived from them. The prosthetic valves which are available for aortic valve replacement in India are all selected according to the BSA of the individual. These standard AV annular diameters which are derived against the BSA, are all indexed only for the western population. But the actual AV diameter for the same BSA may vary between the western population and Indians. Hence, a study on Indian population was undertaken, to compare the aortic valve annulus diameter with respect to the BSA, with the available data of western population.
Material and Methods
This study was conducted on patients who visited the Cardiology Out Patients Department, after getting their consents. The total number of subjects was 406. The subjects who had normal echocardiograms were only taken up for this study. Any other gross or pathological diseases were also ruled out before the subjects were chosen for this study. Each subject’s height in centimetres and weight in kilograms were recorded. This study was conducted on all 406 subjects by doing echocardiograms. The dimensions of annuli of aortic valves were measured by two dimensional echocardiograms, as shown in [Table/Fig-1 and 2]. Echocardiogram which was used in this study was a spatially oriented B mode scan which provided a cross-sectional or a two dimensional image of an object.
RV–Right Ventricle; LV- Left Ventricle; AO–Aorta; LA–Left Atrium
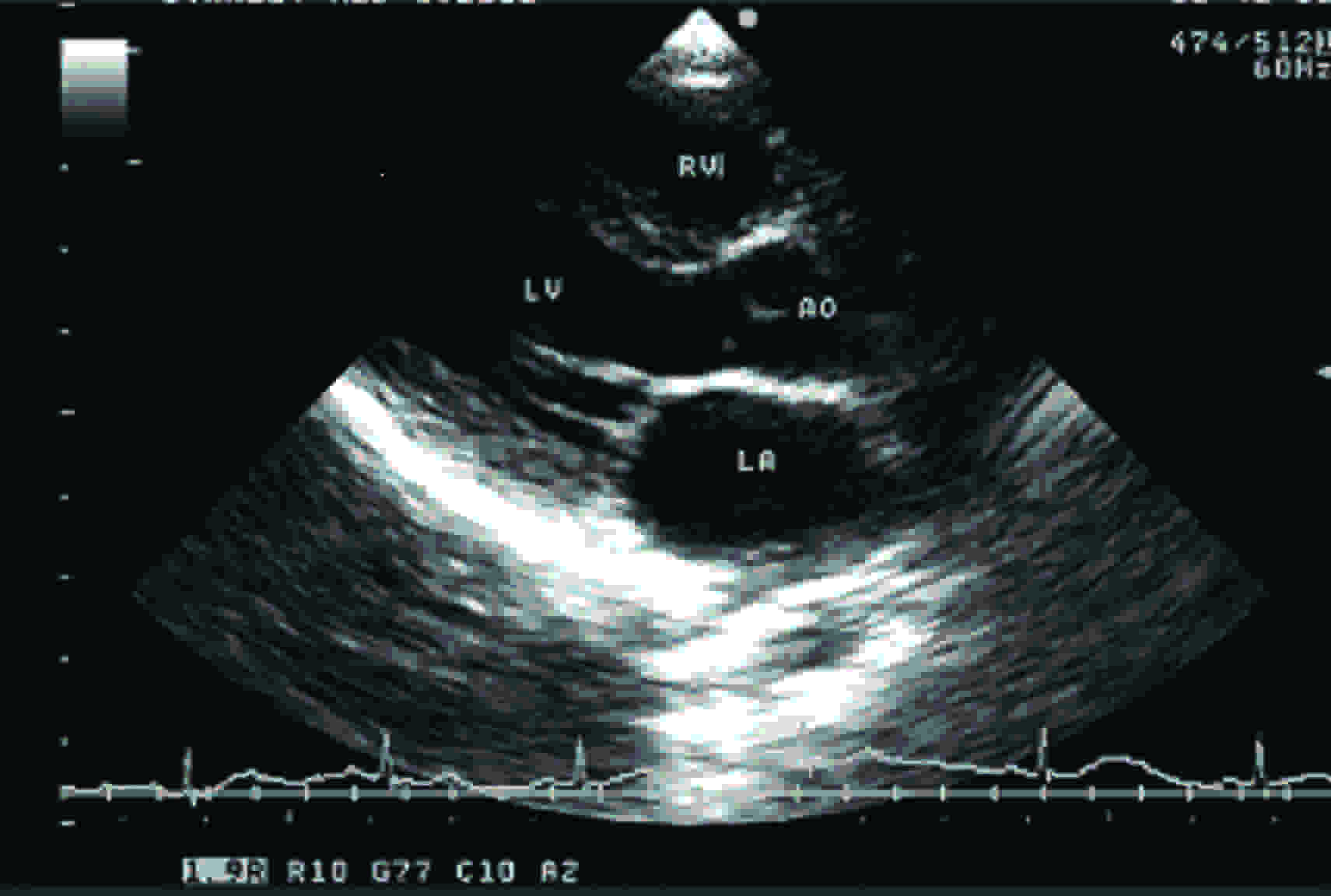
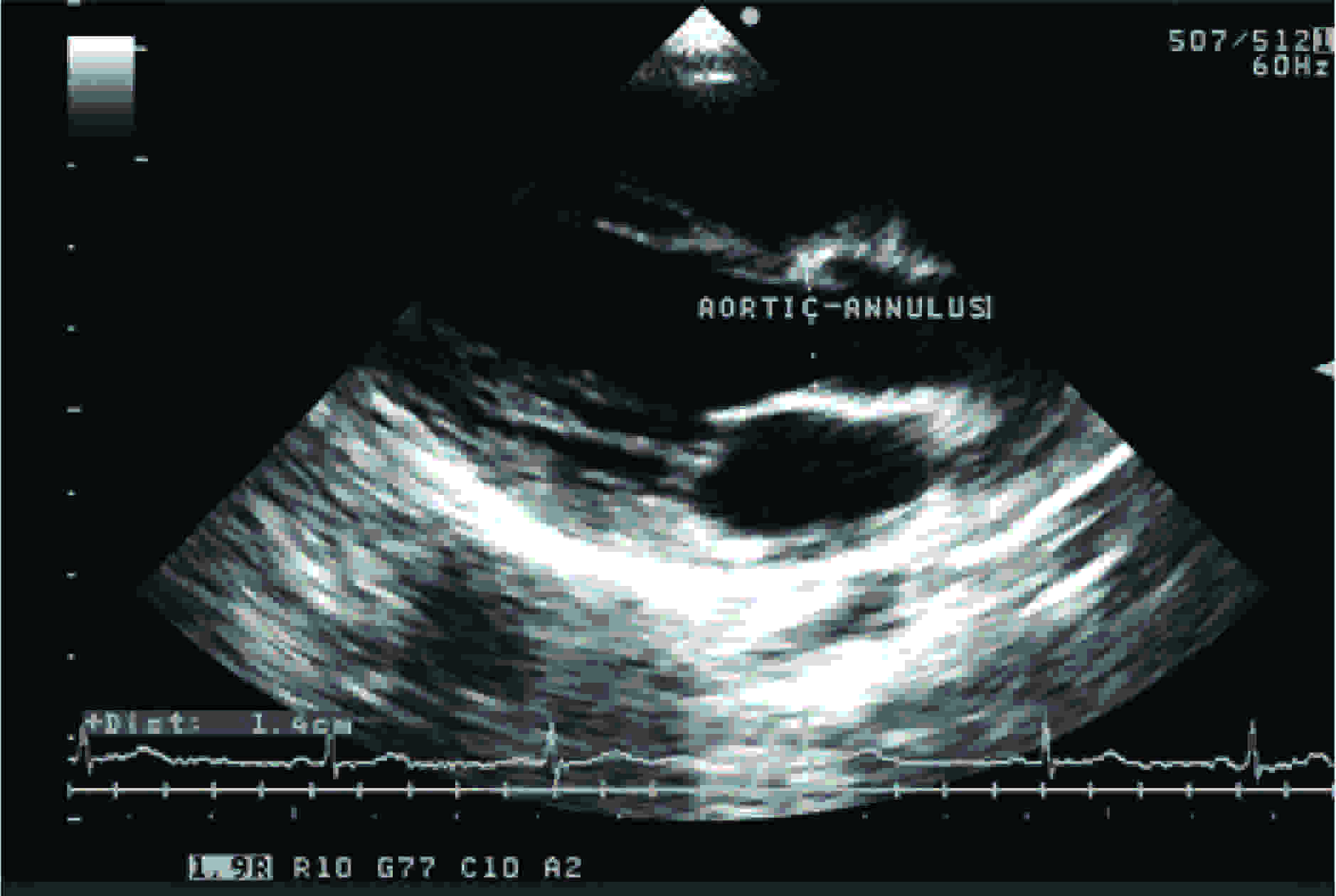
The aortic valve was examined by using the parasternal long axis view during early systole. In this view, the imaging plane transects the aortic valve in an anteroposterior direction and it X-axis is aligned parallel to the long axis of aorta. Because the aorta is normally to the right of the transducer when it is in the parasternal location, the long axis plane is usually oriented, such that it passes through the right and noncoronary aortic leaflets. On the echocardiogram, anteriorly, there are prominent echo signals from the anterior chest wall and anterior right ventricular wall and posteriorly, there are echoes from the posterior wall of the left atrium. The two parallel signals which move synchronously with the cardiac cycle are the echoes from the anterior and posterior walls of the aortic root, respectively. The right ventricular and the left atrial cavities are visualised as relatively echo-free spaces between the anterior right ventricular wall and the posterior wall of the left atrium. In the area which represents the aortic root, echoes are found to originate from the aortic valve cusps. In diastole, these are visualised as a single central line which represents the cusps in the closed position. Frequently, when the gain of the instrument is reduced, the central line is visualised as two or three thin echo signals with a hair line separation (I mm or less). At the onset of ventricular systole, the central line is rapidly replaced by two parallel lines. These latter echoes which lie in close proximity to those from the inner wall of the aortic root represent the aortic valve cusps in the open position. At the onset of ventricular diastole, they come together to fuse and form the central line, thus giving rise to the box-like configuration during systole.
The BSA was calculated by using
Mosteller’s formula: [6]
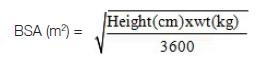
The obtained aortic valve dimensions were categorised according to the BSA and were tabulated. The standard values which were derived from Kirklin/ Barratt- Boyes [6] were compared with the study data. The statistical tests of significance were done for both Indian and standard values of aortic valve annuli. The statistical test of correlation was also done to prove that BSA was a good predictor of the aortic valve dimensions.
Results
The results of all 406 patients were recorded and the average was calculated. The average aortic valve diameter, along with the standard deviation, was computed against the BSA. The BSA values were categorised as were given by Kirklin/ Barratt-Boyes [7]. As observed in [Table/Fig-3], the whole range of aortic valve annulus varied between a minimum of 12.2 mm to a maximum of 21.2 mm in the BSA ranges of 0.6 to 1.9 m2 The aortic valve was examined by using the parasternal long axis view [Table/Fig-1]. Aortic valve shows a linear increase in diameter with increasing BSA, as can be seen clearly in the [Table/Fig-3]. There was an increase of about 2 mm from 0.61-0.7 m2 BSA to 0.71– 0.8 m2 BSA. Afterwards, a linear increase in diameter which ranged from 0.3 to 1mm was seen for body surface area which ranged from 0.81 m2 to 1.2 m2. In the next range of BSA of 1.21 –1.3 m2, there was an increase of 1.5 mm. Then, a steady increase which ranged from 0.4–1 mm was observed in the body surface which ranged from 1.31–1.9 m2.
The mitral valve and body surface area standarized diameters of aortic annulus [mm] BSA: Body Surface Area in m2.
| BSA | Study Population | Standard Values |
|---|
| 0.61 - 0.7 | 12.2 ± 0.4 | 13.5 ± 1.3 |
| 0.71 - 0.8 | 14.1 ± 1.8 | 14.1 ± 1.1 |
| 0.81 - 0.9 | 14. 5 ± 1.5 | 14.6 ± 1.5 |
| 0.91-1.0 | 15.6 ± 1.2 | 15.6 ± 1.3 |
| 1.01 - 1.1 | 16.1 ± 1.3 | 16.3 ± 1.5 |
| 1.11 - 1.2 | 16.6 ± 0.5 | 17.2 ± 1.9 |
| 1.21 - 1.3 | 18.1 ± 1.6 | 17.1 ± 1.6 |
| 1.31 - 14 | 18.5 ± 2.7 | 18.7 ± 1.7 |
| 1.41 - 1.5 | 18.8 ± 1.3 | 19.1 ± 2.3 |
| 1.51 - 1.6 | 19.7 ± 1.5 | 20.7 ± 2.4 |
| 1.61 - 1.7 | 19.8 ± 0.8 | 20.8 ± 2.1 |
| 1.71 - 1.8 | 20.8 ± 0.9 | 21.5 ± 2.0 |
| 1.81 - 1.9 | 21.2 ± 0.9 | 22.3 ± 2.1 |
Discussion
Body surface area, which is a measure of the individual’s height and weight, is used as an index for annular dimensions of cardiac valves. There are studies which have shown a definite correlation between valve annular size and BSA. King DH [8], in 1985, stated that the best predictor for valve annular diameter was a logarithmic function of BSA, with a calculated correlation coefficient which ranged from 0.9–0.93 for 3 annular dimensions. In contrast, Singh B and Mohan JC [9] stated that aortic valve dimension does not correlate directly with the BSA. But the aortic valve dimensions showed a steady increase with increasing BSA [Table/Fig-3] in this study.
Gutgesell HP and French M [10] showed that the valve dimensions were linearly related to BSA. Their data validated the practice of indexing valve area for BSA and for BSA values which ranged from 0.08–2.1m2, the aortic valve diameter was 0.3-2.2 cm and pulmonary valve diameter was 0.4–2.8cm. Indexed mean aortic valve area was 1.33 cm2/m2 and pulmonary valve area was 1.7 cm2/m2 .Similarly, Capps S B et al., [4] showed that the valve diameter directly and significantly correlated with the BSA. King DH [8] showed that best predictor of annular diameter was a logartihmic function of BSA. In the present study, the statistical correlation between BSA and AV diameter was very significant, as can be seen in [Table/Fig-4]. Hence, BSA alone has an excellent correlation with aortic valve dimension area and it is a strong predictor of the aortic valves, as for all other valves. This also proves that the calculation of AV diameter, as against the BSA of the individual, is a reliable method.
| | BSA | AV |
|---|
| BSA | Pearson Correlation | 1 | .842** |
| Sig. (2-tailed) | – | .000 |
| N | 406 | 406 |
| AV | Pearson Correlation | .842 | 1 |
| Sig. (2-tailed) | .000 | – |
| N | 406 | 406 |
***Correlation is significant at the 0.01 level.
According to Pibarot P*, a patient–prosthesis mismatch (PPM) is present when the effective orifice area (EOA) of the inserted prosthetic valve is too small with respect to BSA. (*www.cardiologyonline.com/wchd05/abstracts/3043%20Pibarot.doc) PPM is defined as an EOA which is indexed for BSAs of < 0.8-0.9 cm2/m2 in the aortic position and of < 1.2-1.3 cm2/m2 in the mitral position, where EOA represents the minimal cross-sectional area of the flow jet downstream of the aortic valve (Damien Garcia, Lyes Kadem [11]) This is a frequent problem which is seen in patients who undergo aortic or mitral valve replacements (20–70% prevalence), and its main haemodynamic consequence is the generation of high transvalvular gradients through normally functioning prosthetic valves. The greatest impact of PPM with regards to mortality, is the early postoperative period, especially in patients with depressed LV functions.
Shahbudin H Rahimtoola and Edward Murphy [12], in 1969 –1978, described a condition in which the in vivo prosthetic valve effective orifice area was smaller than that of the native value. Pibarot P and Dimensil JH [13], described that a patient prosthetic mismatch could be present when the effective orifice area of the inserted prosthetic valve is too small with respect to body size. This discrepancy between the inserted prosthetic valves and the original AV diameter is the basic cause of PPM post–operatively. To derive the original annular diameter (a stenosed lesion does not change the annulus where the prosthetic valve needs to be attached), the BSA is used as a predictor. But, the already indexed values of AV diameter, which are derived for the different BSA ranges, are all available only for the western population. Marc R Moon et al., [14], demonstrated that patients with BSA values of greater than 2.1 m2 had a dramatic fall in survival from 78% to 25% with a patient prosthetic mismatch, whereas patients with BSA values of less than 1.7m2 did not experience the same response with a patient prosthetic mismatch. Westaby S et al., [15] gave the mean circularised orifice area in cm2, as shown in [Table/Fig-5].
Mean Circularised Orifice Area in Cm2
| Valve | Male | Female |
|---|
| Aortic | 4.81 ± 1.3 | 3.73 ± 0.98 |
| Pulmonary | 4.88 ± 1.25 | 4.32 ± 1.03 |
| Mitral | 8.7 ± 2.08 | 6.94 ± 1.41 |
| Tricuspid | 11.9 ± 2.12 | 9.33 ± 2.02 |
Comparison of these sizes with the manufacturer’s calculated area for current prosthesis shows that most of the mechanical valves and bioprostheses are potentially restrictive at rest. Tirone E and David MD [16] pointed that small prosthetic aortic valves should be avoided in larger and physically active patients, to reduce the operative risk and to optimise functional recovery and therefore, to prevent a patient prosthesis mismatch. Claudia Blais, Jean G Dumensil and Richard Baillot [17], stressed that a patient prosthesis mismatch was a strong and an independent predictor of short term mortality among patients who underwent aortic valve replacements and that its impact was related both to its degree of severity and the status of left ventricular function.
Hence, a careful selection of bioprosthetic valves with an adequate ratio of effective orifice area to BSA should be ensured. The prosthetic valve sizes which are available are made to the standard values. But there are racial differences to the annular dimensions when they calculated against the BSA. Hence, a study was undertaken in the Indian population. The values of aortic valve dimensions as against the western standards, have been documented in [Table/Fig-3]. In the present study, it was found that there is a very significant difference between Indian and western population in the aortic dimensions, as can be seen in [Table/Fig-6], for BSA values which ranged between 0.61-0.7, 1.11-1.2, 1.21-1.3, 1.51-1.6, 1.61-1.7,1.71-1.8 and 1.8-1.9 m2. The test of significance was done, which compared the Indian and standard values, as can be seen in [Table/Fig-7] and the difference in values was found to be highly significant. In the range of 1.21-1.3 m2 alone, the diameter was larger than standard, whereas in all the other ranges which have been mentioned above, the diameter was smaller than standard values significantly [Table/Fig-7]. When a patient is taken for surgery, the annular dimensions are checked against the patient’s BSA. Thus, this study showed that the normal diameter of aortic valve annulus in Indian population was definitely lower than the standard values in certain ranges of BSA and it also proved that the BSA was an excellent predictor of aortic valve dimension, as there was a linear increase in AV diameter with BSA.
Statistics and One Sample t-test
| BSA | Mean | Std. Deviation | Std. Error Mean | Standard Value | t | df | Sig. |
|---|
| 0.61-0.7 | 12.2000 | .41039 | .09177 | 13.5 | -14.166 | 19 | .000 |
| 0.71-0.8 | 14.1000 | 1.77408 | .39670 | 14.1 | .000 | 19 | 1.000 |
| 0.81-0.9 | 14.4550 | 1.46664 | .32795 | 14.6 | -.442 | 19 | .663 |
| 0.91-1.0 | 15.5650 | 1.18022 | .26391 | 15.6 | -.133 | 19 | .896 |
| 1.01-1.1 | 16.0733 | 1.34773 | .34798 | 16.3 | -.651 | 14 | .525 |
| 1.11-1.2 | 16.6200 | .48433 | .12505 | 17.2 | -4.638 | 14 | .000 |
| 1.21-1.3 | 18.0714 | 1.63069 | .27564 | 17.1 | 3.524 | 34 | .001 |
| 1.31-1.4 | 18.4545 | 2.66540 | .35940 | 18.7 | -.683 | 54 | .498 |
| 1.41-1.5 | 18.8000 | 1.27879 | .21615 | 19.1 | -1.388 | 34 | .174 |
| 1.51-1.6 | 19.6935 | 1.47758 | .18765 | 20.7 | -5.363 | 61 | .000 |
| 1.61-1.7 | 19.8194 | .81061 | .09553 | 20.8 | -10.264 | 71 | .000 |
| 1.71-1.8 | 20.8095 | .87287 | .19048 | 21.5 | -3.625 | 20 | .002 |
| 1.81-1.9 | 21.1875 | .91059 | .22765 | 22.3 | -4.887 | 15 | .000 |
Comparison of Aortic Valve in Indian & Western Population
***Correlation is significant at the 0.01 level.
[1]. Rahimtoola SH, The problem of valve prosthesis-patient mismatchCirculation 1978 58:20-24. [Google Scholar]
[2]. Pibarot Philippe, Dumesnil Jean G, Prevention of valve prosthesis—patient mismatch before aortic valve replacement: does it matter and is it feasible?Heart 2007 May 93(5):549-51. [Google Scholar]
[3]. Habbal ME, Somerville J, Size of the normal aortic root in normal subjects and in those with left venticular outflow obstructionAm J Cardiol 1989 63:322 [Google Scholar]
[4]. Capps SB, Elkins RC, Frank DM, BSA as a predictor of aortic and pulmonary valve diameterJ Thorac Cardiovasc Surg 2000 119:975 [Google Scholar]
[5]. Dan ZHU, Qiang ZHAO, Aortic valve annulus and sinus-tube joint diameters in normal adults of Chinese Han ethnic groupChinese Medical Journal 2008 121(12):1093-95. [Google Scholar]
[6]. Mosteller RD, Simplified Calculation of BSAN Engl J Med 1987 22(317(17)):1098 [Google Scholar]
[7]. Kirklin/ Barratt- Boyes, Cardiac surgery. Third edition. Churchill/ Livingstone. Page No. 37, Table 1B-2 [Google Scholar]
[8]. King DH, Smith EO, Huhta JC, Gutgesell HP, Mitral and tricuspid valve annular diameter in normal children determined by two dimensional echocardiographyAm J Cardiol 1985 55:787 [Google Scholar]
[9]. Singh B, Mohan JC, Atrioventricular valve orifice areas in normal subjectsJnt. J. Cardiol 1994 15(44(1)):85-91. [Google Scholar]
[10]. Garcia Damien, Kadem Lyes, What Do You Mean by Aortic Valve Area: Geometric Orifice Area, Effective Orifice Area, or Gorlin Area?The Journal of Heart Valve Disease 2006 15:601-08. [Google Scholar]
[11]. Gutgesell HP, French M, Echo determination of aortic valve and pulmonary valve in subjects with normal heartsAm J Cardiol 1991 68:773-76. [Google Scholar]
[12]. Rrahimtoola Shahbudin H, Murphy Edward, Valve prosthesis-patient mismatch A long- term sequelaBr Heart J 1981 45:331-35. [Google Scholar]
[13]. Pibarot P, Dumensil JG, Patient prosthesis mismatch definition, clinical impact and preventionHeart 2006 92(8):1022-29. [Google Scholar]
[14]. Moon Marc R, Pasque Michael K, Munfakh Nabil A, Melby Spencer J, Lawton Jennifer S, Moazami Nader, Prosthesis – patient mismatch after aortic valve replacementThe Annals of Thoraic Surgery 2006 81:481-89. [Google Scholar]
[15]. Westaby Karp RB, Blackstone EH, Bishop SF, Adult human valve dimensions and their surgical significanceAm J. Cardiol 1984 53(4):552-56. [Google Scholar]
[16]. Tirone E, David MD, Patient prosthesis mismatchCirculation 2005 111:3186-87. [Google Scholar]
[17]. Claudia Blais, Dumensil Jean G, Baillot Richard, Impact of valve patient prosthesis mismatch on short term mortalityCirculation 2003 108(8):983-88. [Google Scholar]