Evaluation of cardiac blood flows is useful in the haemodynamic management of neonates. Cardiovascular and haemodynamic statuses in neonates are mainly assessed by continuous heart rate monitoring and invasive and non-invasive blood pressure monitoring. Clinical signs like capillary refill time are poorly validated. Indirect markers of tissue perfusion like urine output and serum lactate levels are especially problematic in neonates, due to complex haemodynamic changes which occur during transition to postnatal life [1]. However, these markers are poorly correlated to cardiac blood flows [2, 3].
Cardiac blood flows can be estimated by functional echocardiography by using measures of upper body systemic blood flow which are unaffected by cardiac shunts, such as flow in SVC or by using minimally confounded measures of systemic blood flow like RVO and LVO. The estimates of cardiac blood flow can offer a clearer understanding of the pathophysiology which underlies the various clinical conditions and they may guide in the management of these conditions.
The most commonly measured cardiac blood flows in newborn infants are RVO and LVO and more recently included SVC flow. Studies in preterm infants have shown that an abnormal superior vena cava flow is associated with poor neurodevelopmental outcomes [4]. SGA babies are at a higher risk of neonatal morbidity and mortality and they have poor neurological outcomes. SGA babies are prone to adult onset cardiovascular diseases [5]. Recent studies which were done on foetal growth restriction have postulated alternate pathways for cardiovascular diseases. Foetal growth restriction leads to epigenetic changes in cardiac regulation, leading to persistence of abnormal cardiac function and a lack of ability in further adaptation [5,6].
The incidence of SGA neonates is 5-10% of all live births in developed world, while it is about 25-30% in developing world [7]. In south Asian scenario, where the population of SGA babies is high, it is imperative to have reference values of RVO, LVO, and SVC flows for SGA neonates.
To date, normative data on LVO, RVO and SVC flows has been established for term appropriate for gestational age neonates and preterm neonates, but no data is available on RVO, LVO and SVC flows for term small for gestational age neonates.
With this observational study, we intend to establish the normative data for RVO, LVO and SVC flows in stable term SGA neonates after transitional period.
Methods
This observational study was conducted at Bharati Vidyapeeth University and Medical College, Dhankawadi, Pune, India, from January 2011 to August 2011. Term (37 to 41 weeks gestation) Neonates with birth weights of less than 10th percentile were considered to be eligible for the study.
Informed consents were taken from the parents of all the neonates who were included in the study. The study was approved by the ethics committee of Bharati Vidyapeeth and Medical College, Pune, India.
Echocardiography was performed by PS on day 7 of life of study subjects by using a SIEMENS. Acuson X 300, Siemens Medical Solutions, Inc. USA. machine and a 4-8 MHz transducer.
Exclusion criteria:
Neonates with evidences of congenital infections
Neonates who required respiratory or cardiovascular support and intensive care unit admission for any indication
Neonates with a clinical suspicion of an infection within 48 hours after data collection
Neonates with patent ductus arteriosus or structural heart disease.
Unpaired t-test was used for analysis of flow comparison between symmetric and asymmetric SGA neonates.
Superior vena cava flow measurement: The infants were placed in a supine position on a flat surface and they were studied when they were resting quietly or asleep. The heart was imaged from a low sub-costal view. The SVC flow was identified by angling the beam anteriorly, until the flow from the SVC into the right atrium was seen by using colour Doppler. The angle of insonation was minimized by manoeuvring the transducer inferiorly, to allow visualization of the maximal amount of flow within the SVC before its entry into the right atrium. A pulsed Doppler recording was made at the junction of the SVC and the right atrium. The Doppler range gate was manipulated in the SVC until the clearest ultrasound velocity spectral displays were obtained and a representative sample of 20–30 cardiac cycles was recorded. Values were averaged for 5 cardiac cycles.
The SVC was then imaged on its entering the right atrium, from the parasternal long axis view, with the beam in a true sagittal plane and it was angled to the right of the ascending aorta. The maximum and minimum internal diameters were measured from a frozen image, which showed the vessel walls clearly at the point at which the SVC started to open up into the right atrium, which were obtained from a frame by frame analysis. Because of the variations in vessel diameters throughout the cardiac cycle, a mean of the maximum and minimum diameters within the cardiac cycle was used for calculation of flow.
Diameter measurements of three to five cardiac cycles were averaged.
The SVC flow was calculated by: SVC flow = (velocity time integral × 3.146 × (mean SVC diameter2/4) × heart rate)/body weight.
Right ventricular output: Pulsed Doppler recordings of the flow at the level of the pulmonary valve were made from the parasternal long axis view. An average maximum velocity time integral was derived from the area under the curve, of five consecutive cardiac cycles. The heart rate was measured from the peak to peak intervals of the Doppler velocity time signals. The diameter of the pulmonary valve insertion was measured at end systole from a frame by frame analysis of the 2D parasternal long axis image and the diameters of three to five cardiac cycles were averaged.
Left ventricular output: The LV outflow tract was imaged from an apical view, which was modified to incorporate the full length of the ascending aorta. The pulsed Doppler range gate was placed distal to the aortic valve. The flow velocity time signal was recorded and the maximum velocity time integral was obtained by averaging the values of five consecutive cardiac cycles. The heart rate was measured from the peak to peak intervals of the Doppler velocity time signals. The internal diameter of the ascending aorta, at the site of flow analysis, was measured at the end of systole by using frame by frame analysis of the 2D image, which was taken from a parasternal long axis view. This was obtained by averaging the values of three to five cardiac cycles.
The following formulae were used
Right or left ventricular stroke volume = Velocity time integral × (3.1416X Outflow diameter2/4).
Right or left ventricular output = Stroke volume X Heart rate/Bodyweight (kg).
Results
[Table/Fig-1] Show Flow diagram for inclusion.
Flow diagram for inclusion
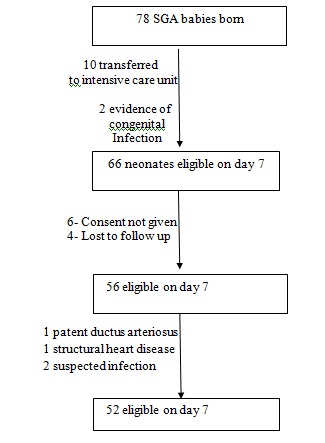
During the 8 month study period, 78 full term SGA infants were born in Bharati Vidyapeeth University and Medical College, Dhankawadi, Pune, India. Fifty two infants were included in the study. The median (range) birth weight was 2.19 kg (1.6-2.41kg).
The characteristics of the study population have been described in [Table/Fig-2].
The characteristics of the study population
| Parameters | Mean± SD |
|---|
| Gestational age (weeks) | 39.75±1.027 |
| Weight (kg) | 2.1906±0.185 |
| Length (cm) | 46.78±1.352 |
| Head circumference (cm) | 33.10±O.926 |
| Ponderal index % | 2.1325±0.176 |
The proportions of symmetric and asymmetric SGA neonates have been depicted in [Table/Fig-3].
Ponderal index distribution
| Ponderal index | Number | Percent |
|---|
| Symmetric | 12 | 23.1 |
| Asymmetric | 40 | 76.9 |
| Total | 52 | 100.0 |
The mean RVO was 255.59 mL/kg/min, mean LVO was 214.61 mL/kg/min and SVC flow was 126.28 mL/kg/min.
The results of central blood flow measurements on day 7 have been described in [Table/Fig-4].
Cardiac blood flow measurements in 52 infants on day 7
| Parameters | Mean ± SD |
|---|
| SVC* VTI† (cm) | 14.49±1.963 |
| SVC diameter (cm) | 0.4165±0.0351 |
| SVC flow (mL/kg/min) | 126.28±31.233 |
| PA‡ VTI† (cm) | 13.075±1.736 |
| PA‡ diameter (cm) | 0.625±0.0464 |
| RVO§ (mL/kg/min) | 255.59±57.429 |
| AVI VTI† (cm) | 10.973±1.8592 |
| Aortic root diameter (cm) | 0.626±0.0498 |
| LVO¶ (mL/kg/min) | 214.61±52.046 |
*Superior vena cava, † velocity time integral, ‡ pulmonary artery, §right ventricular output, II aortic valve, ¶ left ventricular output
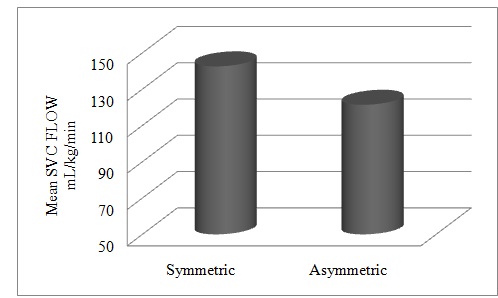
There was a significant difference (p=0.038) between the mean SVC flows of symmetric and asymmetric SGA neonates, but no statistically significant differences were found in RVOs and LVOs of symmetric and asymmetric babies.
[Table/Fig-5] describes the comparison between cardiac flows in symmetric versus asymmetric SGA neonates.
Cardiac blood flow measurements in symmetric and asymmetric neonates
| *SVC Flow | Mean mL/kg/min | Standard Deviation | p |
|---|
| Symmetric | 142.57 | 26.493 | 0.038§ |
| Asymmetric | 121.40 | 31.171 |
| RVO† | | | |
| Symmetric | 251.51 | 58.253 | 0.782 |
| Asymmetric | 256.82 | 57.872 |
| LVO ‡ | | | |
| Symmetric | 221.49 | 52.726 | 0.606 |
| Asymmetric | 212.54 | 52.338 |
* Superior vena cava flow, †Right ventricular output, ‡left ventricular output, § p <0.05 significant difference between SVC flow of symmetric and asymmetric SGA neonates
[Table/Fig-6]: Comparison of SVC flow in symmetric and asymmetric SGA neonates.
Comparison of SVC flow in symmetric and asymmetric SGA neonates Comparison of SVC flow in symmetric and asymmetric SGA neonates (p=0.038)
Discussion
In this study which was done on 52 term SGA neonates, asymmetric SGA neonates constituted 77% of the subjects. Most of the workers from developing countries have reported the preponderance of asymmetric SGA neonates, frequency varying from 51 to 80 [8].
The mean SVC flow was 126.28 mL/kg/min, mean RVO flow was 255.59 mL/kg/min, and mean.
LVO flow was 214.61 mL/kg/min. There was a significant difference (p = 0.038) between the mean SVC flows of symmetric (142.57 mL/kg/min) and asymmetric (121.40 mL/kg/min) SGA neonates. But no significant difference was found between the LVOs and RVOs of symmetric and asymmetric growth restricted neonates. To the best of our knowledge, this is the only study which has provided reference values for the clinically used Doppler derived flows (RVO, LVO and SVC flow) in a cohort of term SGA neonates after the transitional period. Alverson et al., reported an LVO of 236 ± 47 mL/kg/min in 14 term infants who were less than one week of age [9]. Hudson et al., reported an LVO of 231±77 mL/kg/min in 20 healthy term infants [10].
Walther et al., studied 62 term newborn infants during the first weeks of their lives and reported an LVO of 241 ± 33 mL/min/kg [11]. Groves et al., reported an SVC flow of 89 mL/kg/min (54–167) in 13 terms healthy infants at 24 hours of life [12]. Tsai-Goodman et al., reported a mean LVO of 241 mL/kg/min and a mean RVO of 255 mL/kg/min in 24 term healthy infants [13]. Kluckow et al., reported SVC flows in 14 infants who were born after 36 weeks. The median SVC flow rose from 76 mL/kg/min on day 1 to 93 mL/kg/min on day 2 [14]. Lee et al., reported the normal range of SVC flow in the first 2 days of life to be 34–143 mL/kg/min [15]. Groves et al., reported mean (SD) flow volumes which were assessed by phase contrast (PC) imaging in 28 stable term infants. They found an LVO of 222 mL/kg/min (46), an RVO of 219 mL/kg/min (47) and an SVC flow of 95 mL/kg/min (27) [16]. Agata et al., reported LVO in 23 normal term infants to be 258±54 mL/kg/min at 96 hours of life [17]. Agata et al., studied 34 normal term infants, and reported an LV output value of 245±56 mL/kg/min at 24 hours of life, which did not change 96 hours after their births [18].
The values of RVO and LVO in our study were comparable to the values of previously mentioned studies. The values of SVC flow in our study were higher than were previously reported. There may be several reasons for this difference. Firstly, SVC flows which were reported previously were of the first 2 to 4 days of life, during which time, the neonatal haemodynamics was still in transitional phase, while our neonates were studied after this transitional phase. Secondly, the subjects who were included in the previous studies were healthy full term infants and preterm infants, populations which were different from those of our study. Thirdly, recent studies have demonstrated that foetuses and newborns with severe forms of growth restriction have significant changes in their cardiac function parameters [19–21].
In addition, newborns with foetal growth restrictions have increases in their aortic intima-media thicknesses, which supports the existence of vascular remodelling [22].
For deriving normative data, we may require a larger population based study. There is also a need of following up these cardiac blood flow measurements serially, throughout infancy, at regular intervals, to know the correlation of these flows with adult onset cardiovascular disease. Barker’s hypothesis on foetal programming of adult disease mainly emphasizes on SGA neonates and recent studies have demonstrated that foetuses and newborns with severe forms of growth restrictions have significant changes in foetal cardiac function parameters [19–21]. The functional echocardiography parameters in these SGA neonates would be non-invasive clinical parameters, which will help in serial monitoring of their cardiovascular statuses in various disease states.
The establishment of RVO, LVO and SVC flows in preterm and term neonates has helped in the understanding of haemodynamics and pathophysiology of various diseases of these neonates. This study may provide a basis for taking up further cardiovascular and haemodynamic studies on SGA neonates and it has emphasized the importance of recognizing this set of neonates as a special group which is at risk of developing adult onset cardiovascular disease. It also warrants their serial screening throughout infancy and childhood.
Functional echocardiography and measurements of LVO, RVO and SVC flows serially through infancy and childhood may enable us in understanding the haemodynamic statuses of these neonates with respect to the special problems which are faced by them, like hypoglycaemia, polycythaemia and sepsis. The serial measurements of the cardiac blood flows which are made by functional echocardiography may help us in understanding the pathophysiology behind the development of adult onset diseases in SGA neonates.
The main limitation of our study, in deriving the normative data, was the small number of subjects and the limited study duration. We did not evaluate inter and intra-observer variabilities of observations, as all the observations were performed by a single person. Previous data also suggests that all observations should be done by a single observer. No external validity of the observations was done.
*Superior vena cava, † velocity time integral, ‡ pulmonary artery, §right ventricular output, II aortic valve, ¶ left ventricular output
* Superior vena cava flow, †Right ventricular output, ‡left ventricular output, § p <0.05 significant difference between SVC flow of symmetric and asymmetric SGA neonates