Introduction
Maintenance of stable extracellular iron concentrations requires the coordinate regulation of iron transport into plasma from dietary sources in the duodenum, from recycled senescent red cells in macrophages and from storage in hepatocytes. The systemic iron is conserved tightly and its homeostasis thus depends on the regulated expression of hepcidin that negatively regulates iron transport from iron-exporting tissues into plasma, by altering the expression of the ferroportin (cellular iron exporter). Hepcidin acts as a systemic iron-regulatory hormone. This review has focused on the physiology of hepcidin; hepcidin regulation, its correlation with iron stores, erythropoiesis, inflammation, anaemia, hypoxia, cancer and obesity; its correlation with normal iron homeostasis and iron metabolism disorders; the methods for laboratory assessment and finally, diagnostic and therapeutic roles of hepcidin.
Systemic Iron Metabolism
Iron is an essential component of haemoglobin, myoglobin and many enzymes which are involved in energy metabolism. Most of the iron in plasma is directed for erythropoiesis to the bone marrow. A majority of this iron comes from the recycling of senescent erythrocytes by macrophages of the reticuloendothelial system (about 20 mg/day); only 1 to 2 mg of the daily iron supply is derived from intestinal absorption, which, under steady state, is sufficient only to replace the insensible iron loss [1,2]. The erythroid progenitors, because of their large requirement for iron, are dependent upon the transferrin (Tf) cycle.
Structure of Hepcidin
Human hepcidin is a 25–amino acid peptide. The hepatocytes are the main sources of hepcidin [3,4], while the bacteria-activated neutrophils and macrophages are other sources. The structure of the bioactive hepcidin is a simple hairpin with 8 cysteines that forms 4 disulfide bonds in a ladder-like configuration. The urine contains 25–amino acid peptide and also N-terminus truncated 20– and 22–amino acid forms of human hepcidin. The human hepcidin gene contains 84–amino acid preprohepcidin with furin cleavage sites immediately N-terminal to the 25–amino acid major hepcidin [5].
Hepcidin and Ferroportin
The presence of hepcidin in plasma negatively regulates egress of iron from cells which are involved in transport of iron into plasma [1,6,7]. The iron exporter which is required for iron transport from iron exporting cells is Ferroportin 1 (FPN1). FPN1 is a transmembrane protein which transports ferrous iron that must be oxidized to ferric iron by ferroxidases (which also serve in stabilizing FPN1) [8] before iron can bind to Tf. FPN1 is also the receptor for hepcidin. Hepcidin binds to FPN1 which is present on the cell surface, which induces the phosphorylation of FPN1, thus triggering the internalization of the hepcidin-FPN1 complex, leading to the ubiquitinization and lysosomal degradation of both proteins [6,9]. Hepcidin expression restricts iron absorption and macrophage iron release and it reduces body iron stores, thus limiting iron which is available for erythropoiesis.
Hepcidin Kinetics
Circulating hepcidin is bound to α 2-macroglobulin with high affinity and to albumin with a relatively low affinity (11% is freely circulating) [10,11]. Hepcidin clearance occurs via cellular codegradation with ferroportin at its sites of action, and via excretion by the kidneys. In human studies, the fractional excretion of hepcidin has been calculated to be as low as 0–5% [12], either because of reabsorption, or because it was not freely filtered as was evidenced by a 6-fold increase of serum hepcidin concentrations in patients with glomerular dysfunction [13,14] as compared to the 20- to 30-fold increase in serum α 2-microglobulin. Binding to carrier proteins may prevent circulating hepcidin from being freely filtered. Alternatively, a compensatory feedback may decrease hepatic hepcidin production. Under certain conditions, hepcidin may escape renal tubular reabsorption. This lack of reabsorption may play a role in several disorders of iron metabolism that are associated with tubular dysfunction and increased concentrations of urine hepcidin, such as inflammation, iron overload, and malaria [10].
Regulation of Hepcidin
At present, four pathways control liver hepcidin production: (i) iron store-related regulation (ii) erythropoietic activity driven regulation (iii) inflammation related regulation, and (iv) mandatory signaling pathway. All interact with hepatocytes to initiate/inhibit the production of sufficient hepcidin for iron homeostasis [15–17].
Regulation of Hepcidin Synthesis by Iron
Studies which were done on haemochromatosis patients with defects in hepcidin regulation have suggested the involvement of a pathway which involved the proteins, haemojuvelin and transferrin receptor [2]. The receptor for bone morphogenic protein (BMP) may also be important for the regulation of hepcidin by iron and other stimuli, as was suggested by the ability of haemojuvelin to potentiate BMP signaling by the strong stimulatory effects of bone morphogenetic proteins, 2, 4 and 9 on hepcidin synthesis. The human hepatocytes in culture also respond to inflammatory stimuli of increasing hepcidin mRNA [3].
Regulation of Hepcidin Synthesis by Anaemia and Hypoxia
Haemoglobin production is increased following erythropoietic stimuli such as blood loss, anaemia or hypoxia. Hence, hepcidin production is also homeostatically regulated by them. During hypoxia, the homeostatic response produces more erythrocytes and thus, hepcidin levels decrease; hepcidin inhibitory effects diminish and thus, more iron is made available from diet and from the storage pool. The hepcidin response to anaemia could be mediated by tissue hypoxia, increased erythropoietin levels and an increased erythropoietic activity. Studies have pointed to erythropoietic activity as the most potent suppressor of hepcidin synthesis [3,18].
Hepcidin and Erythropoiesis
Iron-loading anaemias are characterized by ineffective erythropoiesis and increased intestinal iron absorption. The most common iron-loading anaemias are the intermediate and major forms of β-thalassaemia, and the other rare anaemias include congenital dyserythropoietic anaemia, X-linked sideroblastic anaemia and anaemia which is associated with DMT1 mutation. In the presence of a systemic iron overload, urinary hepcidin concentrations are low, which suggest the dominant effect of erythropoietic drive as a suppressor of hepcidin synthesis [3, 19].
Hepcidin and Inflammation
Anaemia of inflammation is a common consequence of chronic infections, noninfectious generalized inflammatory disorders and some cancers, and it can also develop during sepsis. These anaemias are characterized by decreased serum iron and iron-binding capacities (transferrin), increased ferritin, and the presence of iron in bone marrow macrophages, which indicate an impaired mobilization of iron from stores. The link between infections, hypoferraemia and anaemia of inflammation suggests that they are a part of host defense responses to infection. Patients with hypoferraemia and anaemia which are caused by infections or inflammatory disorders have increased urinary hepcidin excretion. Patients who are on experimental IL-6 therapy and diseases which are associated with IL-6 excess such as Castleman’s syndrome, multiple myeloma and juvenile rheumatoid arthritis also develop anaemia. Thus, the proposed pathogenic cascade that produces anaemia of inflammation leads IL-6 and possibly other inflammatory cytokines to hepcidin to hypoferraemia, and then to anaemia of inflammation [3, 20].
Mechanism of Action of Hepcidin
Depending on the cell type, iron can be taken up by several distinct pathways. Bioavailable iron in the diet is mostly present either in its ferric form or as heme. The uptake of ferric iron is mediated by a combination of ferric reductase, which reduces iron to its ferrous form, and a ferrous iron transporter, DMT1, that moves iron across the cell membrane. A heme transporter, the apical heme carrier protein 1, is also found in duodenal enterocytes, [21]. Macrophages that recycle iron from senescent erythrocytes first phagocytoze and lyse the erythrocytes and they then extract the iron from heme by using heme oxygenase. Other cells import iron through transferrin receptors that capture and endocytoze diferric transferrin, and the low vacuolar pH removes ferric iron from the transferrin–transferrin receptor complex. The transport of iron across vacuolar membranes into the cytoplasm of macrophages involves DMT1, which is specific for ferrous iron. The necessary iron reduction is catalyzed by an erythroid endosomal ferric reductase, which is important for iron utilization in erythrocytes [22]. In the cytoplasm, iron which is is stored is bound to ferritin.
Ferroportin, the cellular exporter of iron, is expressed on cells that act as iron handlers in the body–duodenal enterocytes which absorb dietary iron, macrophages in liver and spleen which recycle old erythrocytes, hepatocytes which store iron and placental trophoblasts which transfer iron to the foetus during pregnancy [23,24], and on erythroid precursor cells. Hepcidin acts by modulating cellular iron export through ferroportin to plasma and extracellular fluid [Table/Fig-1].
Hepcidin synthesis and release by the liver is regulated by plasma iron concentration, erythropoietic activity and inflammation. The hepcidin inturn interacts with ferroportin (Fpn). This hepcidin- ferroportin interaction inturn modulates cellular iron export to plasma and extracellular fluid
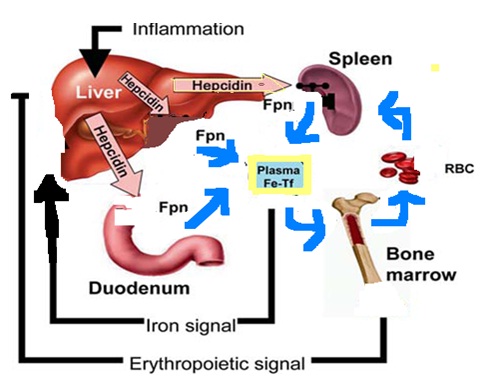
When iron stores are adequate or high, the liver produces hepcidin, which circulates to the small intestine. There, hepcidin causes ferroportin to be internalized, thus blocking the pathway for the transfer of iron from enterocytes to plasma and from macrophages. When iron stores are low, hepcidin production is suppressed. Ferroportin molecules are expressed on basolateral membranes of enterocytes, and they transport iron from enterocyte to plasma transferrin. The hepcidin-ferroportin interaction explains the regulation of macrophage recycling of iron and also the presence of iron-containing macrophages in inflammatory states, which are characterized by a high production of hepcidin [21].
Clinical Applications of Hepcidin
Many disorders are associated with inadequate/deficiency in hepcidin production and with excess hepcidin levels in body. Some of them have been enumerated in [Table/Fig-2].
Iron disorders associated with inappropriate hepcidin production
| Defect in Hepcidin producton | Disease |
|---|
| Hepcidin Deficiency | Hereditary hemochromatosis Iron-loading anemia, Hepatitis C infection |
| Hepcidin Excess | Anaemia of inflammation, Chronic kidney disease (CKD), Iron refractory iron deficiency anemia |
Diseases of Hepcidin Deficiency
Hepcidin deficiency results in the development of a systemic iron overload which is caused by excessive iron absorption. In the absence of hepcidin, ferroportin expression on the basolateral surface of enterocytes is increased, thus increasing transport of dietary iron into plasma. In hepcidin deficiency, macrophages also display increased ferroportin on their cell membranes and they thus export more iron. Excess plasma iron accumulates in organs, as the rate of iron uptake exceeds the rate of iron export. The liver is most commonly affected by iron overload, due to the increased uptake of non-Tf-bound iron by hepatocytes. In iron-loading anaemias such as β-thalassaemia and congenital dyserythropoietic anemias, urinary and serum hepcidin is severely decreased despite systemic iron overload [21, 25] which may be due to the high erythropoietic activity that suppresses hepcidin synthesis, which thus outweighs the effects of iron overload on hepcidin regulation.
Hepcidin in Hereditary Haemochromatosis
Hepcidin levels in juvenile hemochromatosis are extremely low. Less decreased hepcidin levels have been found in TfR2 mutations. The presence of iron overload at presentation and the increased erythropoiesis with phlebotomy treatment may contribute to the mechanism behind this observed variability in hepcidin values. In ferroportin disease, hepcidin concentrations appear to vary with the sequence variations of the gene and the way they influence the activity of the ferroportin protein. Patients with 162 del Val and N144H alterations in ferroportin were reported to have high hepcidin levels, with a loss of function of ferroportin [26, 27].
Hepcidin in Acquired Forms of a Non-Haemochromatotic Iron Overload
Acquired liver diseases like excessive alcohol consumption, nonalcoholic fatty liver disease and nonalcoholic steatohepatitis are associated with a mild hepatic iron overload. In non-haemochromatotic iron overload diseases, hepcidin concentration is increased, but it is relatively low for the level of iron overload, due to an ineffective liver iron sensing. Here, dysregulation of hepcidin synthesis, which is a consequence of reactive oxygen species, endoplasmic reticulum stress and cytokine tumour necrosis factor-α–mediated pathways, may contribute to a decreased hepcidin synthesis and iron accumulation [28, 29]. Transfusions increase hepcidin levels, presumably due to both the alleviation of an ineffective erythropoiesis and an increased iron load. Hepatitis C is frequently accompanied by a hepatic iron overload which worsens liver damage and low hepcidin levels. The mechanism may include a virus-induced oxidative stress which suppresses hepcidin synthesis [10].
Hepcidin in Iron Deficiency Anaemia (IDA)
In IDA, in patients with low ferritin concentrations without anaemia, as is seen secondary to severe blood loss and an insufficient dietary intake, hepcidin concentrations are very low [10].
Effect of Iron Administration on Hepcidin Concentrations
In healthy humans, urine and serum hepcidin increased upon oral iron administration, although nonresponders were observed in many studies. Few studies reported a weak, but a significant negative correlation between serum hepcidin and iron absorption [30]. Thus, serum hepcidin concentration could be a predictor of the therapeutic effect of oral iron administration and it might prove to be useful for evaluating iron absorption in iron deficiency.
Diseases which are Caused by Excess Hepcidin
In inflammatory disorders, hepcidin production is stimulated by increased cytokines, prominently IL-6. A chronic hepcidin-mediated iron restriction may eventually lead to Anaemia of Inflammation (AI) [31]. Elevated hepcidin levels are observed in rheumatologic diseases, inflammatory bowel disease, multiple myeloma and critical illnesses. Animal studies have indicated that increased hepcidin causes iron restriction and a blunted erythropoietic response to erythropoietin (EPO), which are characteristic of AI. Hepcidin does not appear to decrease red blood cell survival, which is another feature which is associated with AI [21].
Hepcidin concentrations were reported to be increased in CKD patients [32]. CKD patients with or without significant inflammation had elevated hepcidin levels, which progressively increased with the severity of CKD. Hepcidin is partly cleared by renal filtration; decreased kidney functions may contribute to this phenomenon. Serum hepcidin is increased in end-stage renal disease patients, which could be partly reduced by haemodialysis. It has been postulated that high hepcidin levels may be the reason for EPO resistance which is commonly observed in CKD patients, which needs further studies to confirm it. Much of the aetiology behind renal anaemia is still unclear and other factors such as hyperparathyroidism, aluminum toxicity, systemic inflammation, and impaired iron metabolism, may be of minor importance [21].
IRIDA is a disease which is characterized by congenital hypochromic, microcytic anaemia, which is refractory to treatment with oral iron, and only partially responsive to parenteral iron. IRIDA is caused by increased hepcidin production which is caused by mutations in the hepcidin suppressor, TMPRSS [6,15].
Hepcidin in Renal Diseases
The introduction of Erythropoiesis Stimulating Agents (ESA) such as EPO, has allowed effective treatment of anaemia in patients with CKD. But many patients become ESA resistant. Hepcidin has been recognized as potentially relevant in CKD, because it may be responsible for the imbalance between iron homeostasis and ESA resistance [33]. Hepcidin concentrations in CKD patients were increased as compared to those in healthy controls [34,35]. Almost all studies which were done on hepcidin in CKD patients have revealed a strong positive relationship between hepcidin and ferritin concentrations. Some studies have demonstrated correlations between hepcidin and iron or transferrin saturations [34]. Few studies have associated Hepcidin in CKD with iron-restricted erythropoiesis, as was reflected by the correlation of hepcidin with lower haemoglobin levels and/or reticulocyte counts. CRP and IL-6 were less relevant predictors in the presence of renal insufficiency. Intravenous iron administration did not influence hepcidin concentrations in haemodialysis (HD) patients who were withheld from iron and ESA therapy for two weeks, but it increased hepcidin in iron-naıve CKD patients [34]. Studies have also reported a decrease in hepcidin concentrations after EPO administration. One study revealed that hepcidin concentrations in EPO responders did not differ from those of low-responders, but cross-sectional studies which were done among haemodialysis patients and a prospective study which was done among patients with combined CKD and chronic heart failure revealed that nonresponders had low hepcidin concentrations [32]. Hepcidin can thus be regarded as a marker of response rather than resistance to ESA treatment in these patients. Though hepcidin can be a companion which is diagnostic of ESA therapy, patients with renal insufficiency are a complex population who pose a difficulty in obtaining consistent results. Clinical stability, time of sampling with respect to iron and ESA therapy, and dialysis regimens differ between studies and they are likely to influence results. Hence, further, larger, well designed studies on hepcidin concentrations in CKD are necessary. As a marker of iron metabolism in CKD patients, it may also serve as a marker of renal disease itself. Studies have suggested that a smaller increase in urinary hepcidin which is relative to serum hepcidin may be associated with a greater risk of acute kidney injury after coronary artery bypass graft surgeries in patients with stable renal functions [35]. In a study which was done on urine biomarkers in patients with lupus nephritis, urinary concentrations of hepcidin, which was possibly secreted by interstitial inflammatory cells, were identified as markers of renal lupus nephritis flare.
Hepcidin in Obesity-Related Diseases
Adipose tissue is an active endocrine organ that releases several cytokines and adipokines which contribute to development of low-grade systemic inflammation. It can also produce hepcidin [36]. The low-grade inflammation in obese and dysmetabolic iron overload syndrome patients (which is characterized by the association of increased body iron stores and metabolic features) is associated with increased hepcidin concentrations, leading to a poor iron absorption and causing Anaemia of Chronic Disease (ACD) [37]. In individuals with weight loss, serum hepcidin concentrations are decreased, with an increase in iron status and intestinal absorption [38]. In chronic mild inflammatory conditions such as obesity or the metabolic syndrome, even a mild hepcidin excess may alter the balance between iron loss and iron uptake, leading to an iron deficiency.
Hepcidin in Cancer
Patients with cancer often have anaemia, generally in association with other markers of inflammation. Studies which were done on patients with multiple myeloma and Hodgkin’s lymphoma, suggest that hepcidin is upregulated by IL 6–dependent and IL 6 –independent mechanisms that may play a role in the anaemia which is often seen in these patients [39,40]. The IL 6–independent pathway may be formed by increased BMP-2 concentrations, which have been suggested to act synergistically with IL-6, to induce hepatocyte hepcidin synthesis. In a study which was done on anaemic cancer patients who received ESA therapy, those with relatively low hepcidin concentrations at baseline, showed a better response [41]. The baseline hepcidin concentrations may play a potential role as a predictive marker for identifying patients who will respond adequately to EPO therapy or who need to be excluded from anaemia correction with ESA, owing to a low chance of responding. Future studies are needed to evaluate the position of hepcidin in clinical decision making in these patients.
Assessment of Urinary and Serum Hepcidin
Hepcidin mRNA expression assay is preferred in animal and cell culture studies, but it is rarely used in human studies, because of invasive sampling. Immunochemical methods which are based on the use of specific anti-hepcidin antibodies such as which are used in immuno-histochemical tissue staining, SDS-PAGE and western blot, have the disadvantage of a limited availability of suitable antibodies [42]. This may be due to small size of hepcidin, compact and complex structure of the molecule, and highly conserved sequence among species, which complicate the elicitation of an immune response in host species [15].
Antibody-Based Hepcidin Assay
An antibody-based dot blot assay can be used to semi-quantify hepcidin in urine. Hepcidin can be quantified by chemiluminescence by using rabbit anti-human hepcidin primary antibodies, and goat anti-rabbit horseradish peroxidase as a secondary antibody. Here, urine hepcidin is normalized by using urinary creatinine concentrations. The limited availability of noncommercially made antibodies makes optimization of antibody-based assays difficult, with a guaranteed specificity. Hepcidin ELISA increases the accessibility of the analysis, but it does not discriminate between the different isoforms. Measurement of pro-hepcidin can be done by using ELISA. Its diagnostic use is controversial due to lack of a clear correlation with hepcidin and other iron related parameters, but significant concentration differences have been reported only in ferroportin disease or in combination with end-stage renal disease [15].
Mass Spectrometry-Based Hepcidin Assay
Surface-enhanced, laser desorption/ionization time-of-flight mass spectrometry based assays can detect the isoforms of hepcidin. Serum hepcidin can be analyzed by using liquid chromatography and tandem mass spectrometry, with the use of a non-hepcidin related internal standard. Both need highly specialized equipments. Presently, mass spectrometry can be used to semi-quantify serum and urine hepcidin levels in clinical research studies [15].
Pre-Analytical Factors and Diurnal Variation
Pre-analytical factors substantially influence, especially on urinary hepcidin, and a large diurnal variation in serum hepcidin levels. Murphy et al., observed no major differences between serum concentrations among normal males and females [43].
Hepcidin-Modulating Agents
Hepcidin-targeted therapies may improve treatment options for patients who suffer from iron disorders. Several compounds are under development as hepcidin agonists or antagonists [44]. Hepcidin agonists could be useful for preventing an iron overload which is caused by a hepcidin deficiency, such as that which is seen in HH, β-thalassaemias and other iron-loading anaemias, and in some acquired forms of nonhaemochromatotic iron-overload diseases. Hepcidin antagonists may benefit patients with diseases of hepcidin excess, such as iron-refractory IDA, ACD (rheumatic diseases, inflammatory bowel diseases and autoimmune diseases), CKD, multiple myeloma and other cancers, obesity-related iron deficiency and cardiovascular disease [10].
Clinical Applications of Hepcidin
Diagnostics
Considering that hepcidin is fundamentally involved in the pathogenesis of many iron disorders, its measurement in biological fluids may facilitate the diagnosis of those diseases [Table/Figure 2]. Many assays which include competitive ELISAs and mass-spectrometry-based assays have been developed. The utility of hepcidin in the diagnosis and prognosis of iron disorders needs to be evaluated in larger clinical studies [21].
Therapeutics
As most types of hereditary haemochromatosis are caused by hepcidin deficiency, hepcidin agonists may be useful. This was found to be true in animal studies. In patients with an advanced iron overload at diagnosis, hepcidin agonists may be useful as adjuncts to therapeutic phlebotomy, where they may redistribute iron to macrophages and away from parenchymal cells, block further iron absorption and eventually, eliminate the need for a maintenance phlebotomy. A similar approach may be useful in hepatitis C patients with a hepatic iron overload. In β-thalassaemias and other iron-loading anaemias, hepcidin agonists may help in controlling intestinal iron absorption which is the sole cause of iron overload in patients who have not received transfusion. For patients with a transfusion- related iron overload, the utility of hepcidin agonists is not clear, but it is possible that hepcidin could alter the distribution of iron from parenchyma to macrophages, where it is less harmful. Hepcidin antagonists may benefit patients with diseases of hepcidin excess. Apart from those which directly interfere with hepcidin activity, like hepcidin-neutralizing antibodies or hepcidin siRNA, other agents which target pathways which regulate hepcidin production have been described. Dorsomorphin, an inhibitor of BMP signaling, prevents hepcidin induction by iron in vivo [21]. Soluble HJV, an antagonist of BMP signaling, decreases hepcidin baseline expression in mice and it concurrently increases liver iron content. In patients with inflammatory diseases, anaemia may be responsive to anti-cytokine therapies such as anti-IL-6 antibodies. Future studies are needed to assess the risks and relative benefits of these treatment approaches.
Conclusion
Hepcidin is the key regulator of iron balance, and high hepcidin levels cause iron blockade and anaemia in chronic disease. Hepcidin is a promising diagnostic tool, but efforts must be undertaken to assess the relevance of specifically measuring hepcidin- 25, to harmonize assay outcomes throughout the world, to define clinical decision limits, and to make assays available to clinical laboratories, before hepcidin assays can be fully included in clinical practice. These advances will improve the health and well-being of many millions of patients with iron disorders. Hepcidin and its regulatory pathways are potential therapeutic targets which could lead to an effective treatment of anaemia in chronic disease.
[1]. Fleming Mark D, The Regulation of Hepcidin and Its Effects on Systemic and Cellular Iron MetabolismHematology 2008 :151-58. [Google Scholar]
[2]. Andrews NC, Disorders of iron metabolismN Engl J Med 1999 341:1986-95. [Google Scholar]
[3]. Ganz Tomas, Hepcidin and Its Role in Regulating Systemic Iron MetabolismHematology 2006 :29-35. [Google Scholar]
[4]. Park CH, Valore EV, Waring AJ, Ganz T, Hepcidin, a urinary antimicrobial peptide synthesized in the liverJ Biol Chem 2001 276:7806-10. [Google Scholar]
[5]. Tomosugi N, Kawabata H, Wakatabe R, Detection of serum hepcidin in renal failure and inflammation by using Protein Chip SystemBlood 2006 108:1381-87. [Google Scholar]
[6]. Lesbordes-Brion JC, Viatte L, Bennoun M, Targeted disruption of the hepcidin 1 gene results in severe hemochromatosisBlood 2006 108:1402-05. [Google Scholar]
[7]. Yamaji S, Sharp P, Ramesh B, Srai SK, Inhibition of iron transport across human intestinal epithelial cells by hepcidinBlood 2004 104:2178-80. [Google Scholar]
[8]. De Domenico I, Ward DM, di Patti MC, Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasminEMBO J 2007 26:2823-31. [Google Scholar]
[9]. De Domenico I, Ward DM, Langelier C, The molecular mechanism of hepcidin-mediated ferroportin downregulationMol Biol Cell 2007 18:2569-78. [Google Scholar]
[10]. Kroot Joyce JC, Tjalsma Harold, Fleming Robert E, Swinkels Dorine W, Hepcidin in Human Iron Disorders: Diagnostic ImplicationsClinical Chemistry 2011 57:12:1650-69. [Google Scholar]
[11]. Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in bloodBlood 2009 113:6225-36. [Google Scholar]
[12]. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M, Immunoassay for human serum hepcidinBlood 2008 112:4292-97. [Google Scholar]
[13]. Peters HP, Laarakkers CM, Swinkels DW, Wetzels JF, Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rateNephrol Dial Transplant 2010 25:848-53. [Google Scholar]
[14]. Costa E, Swinkels DW, Laarakkers CM, Rocha-Pereira P, Rocha S, Reis F, Hepcidin serum levels and resistance to recombinant human erythropoietin therapy in haemodialysis patientsActa Haematol 2009 122:226-29. [Google Scholar]
[15]. Kemna Erwin HJM, Tjalsma Harold, Willems Hans L, Swinkels Dorine W, Hepcidin: from discovery to differential diagnosisHaematologica 2008. Jan 93(1):90-97. [Google Scholar]
[16]. Babitt JL, Huang FW, Wrighting DM, Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expressionNat Genet 2006 38:531-39. [Google Scholar]
[17]. Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S, Suppression of hepcidin during anemia requires erythropoietic activityBlood August 1, 2006 as Epub ahead of print [Google Scholar]
[18]. Nicolas G, Chauvet C, Viatte L, The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammationJ Clin Invest 2002 110:1037-44. [Google Scholar]
[19]. Papanikolaou G, Tzilianos M, Christakis JI, Hepcidin in iron overload disordersBlood 2005 105:4103-05. [Google Scholar]
[20]. Weinstein DA, Roy CN, Fleming MD, Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic diseaseBlood 2002 100:3776-81. [Google Scholar]
[21]. Nemeth Elizabeta, Ganz Tomas, Geffen David, The Role of Hepcidin in Iron MetabolismActa Haematol 2009 November 122(2-3):78-86. [Google Scholar]
[22]. Ohgami RS, Campagna DR, Greer EL, Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cellsNat Genet 2005 37:1264-69. [Google Scholar]
[23]. Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, The iron exporter ferroportin/Slc40a1 is essential for iron homeostasisCell Metab 2005 1:191-200. [Google Scholar]
[24]. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalizationScience 2004 306:2090-93. [Google Scholar]
[25]. Nemeth E, Ganz T, Hepcidin and iron-loadin ganemiasHaematologica 2006 91:727-32. [Google Scholar]
[26]. Papanikolaou G, Tzilianaos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Hepcidin in iron overload disordersBlood 2005 105:4103-05. [Google Scholar]
[27]. Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T, The Nterminus of hepcidin is essential for its interaction with ferroportin: structure-function studyBlood 2006 107:328-33. [Google Scholar]
[28]. Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, ER stress controls iron metabolism through induction of hepcidinScience 2009 325:877-80. [Google Scholar]
[29]. Harrison-Findik DD, Role of alcohol in the regulation of iron metabolismWorld J Gastroenterol 2007 13:4925-30. [Google Scholar]
[30]. Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF, Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidinAm J Clin Nutr 2009 90:1280-87. [Google Scholar]
[31]. Agarwal N, Prchal JT, Anemia of Chronic Disease (Anemia of Inflammation)Acta Haematol 2009 122:103-08.(DOI: 10.1159/000243794) [Google Scholar]
[32]. Silverberg D S, Wexler D, Palazzuoli A, Iaina A, Schwartz D, The Anemia of Heart FailureActa Haematol 2009 122:109-19.(DOI: 10.1159/000243795) [Google Scholar]
[33]. Swinkels DW, Wetzels JF, Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease?Nephrol Dial Transplant 2008 23:2450-53. [Google Scholar]
[34]. Weiss G, Theurl I, Eder S, Koppelstaetter C, Kurz K, Sonnweber T, Serum hepcidin concentration in chronic haemodialysis patients: associations and effects of dialysis, iron and erythropoietin therapyEur J Clin Invest 2009 39:883-90. [Google Scholar]
[35]. Prowle JR, Westerman M, Bellomo R, Urinary hepcidin: an inverse biomarker of acute kidney injury after cardiopulmonary bypass?Curr Opin Crit Care[Epub ahead of print 2010 Aug 21] [Google Scholar]
[36]. Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASHGastroenterology 2006 131:788-96. [Google Scholar]
[37]. Aeberli I, Hurrell RF, Zimmermann MB, Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight childrenInt J Obes 2009 33:1111-17. [Google Scholar]
[38]. Amato A, Santoro N, Calabro P, Grandone A, Swinkels DW, Perrone L, Effect of body mass index reduction on serum hepcidin levels and iron status in obese childrenInt J Obes 2010 34:1772-74. [Google Scholar]
[39]. Maes K, Nemeth E, Roodman GD, Huston A, Esteve F, Freytes C, In anemia of multiple myeloma, hepcidin is induced by increased bone morphogenetic protein 2Blood 2010 116:3635-44. [Google Scholar]
[40]. Hohaus S, Massini G, Giachelia M, Vannata B, Bozzoli V, Cuccaro A, Anemia in Hodgkin’s lymphoma: the role of interleukin-6 and hepcidinJ Clin Oncol 2010 28:2538-43. [Google Scholar]
[41]. Ukarma L, Johannes H, Beyer U, Zaug M, Osterwalder B, Scherhag A, Hepcidin as a predictor of response to epoetin therapy in anemic cancer patientsClin Chem 2009 55:1354-60. [Google Scholar]
[42]. Dallalio G, Fleury T, Means RT, Serum hepcidin in clinical specimensBr J Haematol 2003 122:996-1000. [Google Scholar]
[43]. Murphy AT, Witcher DR, Luan P, Wroblewski VJ, Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometryBlood 2007 in press [Google Scholar]
[44]. Pietrangelo A, Hepcidin in human iron disorders: therapeutic implicationsJ Hepatol 2011 54:173-81. [Google Scholar]