A 75–years old man was hospitalized with symptoms which suggested gastric cancer. Thirty-eight years ago, he had undergone a Billroth-II gastric reconstruction for a peptic ulcer. At the present admission, he had presented with an eight-month history of recurrent haematemesis, epigastric pain, vomiting, and fatigue. The emergent endoscopy showed a type 0-IIc (superficial depressed) early gastric stump cancer in the anastomotic area and total removal of the gastric remnant and the jejunal segment was performed. The histological examination of the surgical specimen showed a gastric adenocarcinoma that invaded the mucosa and the submucosa, without lymph node metastases (pT1bN0 stage). Besides the tumour, enlarged vessels were observed in the submucosa and the muscularis propria, some of which were thrombotic. The surrounding normal gastric wall also presented submucosal oversized vascular spaces, some of which were protruding through the muscularis mucosae in the mucosal layer. Based on these characteristics and the recurrent haematemesis, a final diagnosis of early gastric stump carcinoma which was associated with Dieulafoy’s lesion was made. This association has not yet been reported in the literature and it allowed us to diagnose the gastric stump cancer in a very early stage.
Introduction
Dieulafoy’s lesion is a rare lesion of the gastrointestinal tract, that can produce severe and even fatal haemorrhages. It is either diagnosed at endoscopy or under a microscope, and its rarity is rather because of a lack of recognition than the true rarity. This lesion, which is also known as a gastric caliber-persistent artery, was first described in 1898 by Dieulafoy, as “exulceratio simplex” [1–3]. It is considered to be a vascular malformation of the left gastric artery, that consists of the persistence of the arterial diameter in the gastric wall, but the real pathogenesis is still under debate. Macroscopically, it is either an ulcerated or polypoid lesion which is usually localized within 6 cm of the proximal stomach, on the lesser curvature [1,4] but it has also been described in the oesophagus, duodenum, ileum, and the colon [3]. Under the microscope, a protruded or an ulcerated lesion with spurting arteries in its base is observed. Abnormally dilated vascular spaces are seen in the submucosa and the mucosa, and their erosion can produce severe bleedings [5,6].
In some cases, Dieulafoy’s lesion can masquerade other lesions such as a gastric cancer, that can facilitate the cancer diagnosis in the early stages. In this paper, we are presenting an early gastric stump cancer (GSC) which was diagnosed 38 years after a distal gastrectomy was performed for a gastric ulcer. The particularity of this case lies in its association with Dieulafoy’s lesion. To date, about 17 cases of gastric carcinomas (primary gastric cancers) which were associated with Dieulafoy’s lesions have been reported, the last one being reported in 2006 [5]. However, most of them were published in Japanese and only three were reported in the English literature [1,4,5]. This is the first case report that has revealed the association between a GSC and a Dieulafoy’s lesion.
Case Presentation
A 75-years old normostenic man (his body weight was 65 kg and his height was 1.63 m) who underwent distal gastrectomy with a Billroth–II reconstruction 38 years ago for a peptic ulcer which was located in the antrum, was admitted to our hospital with an 8–month history of recurrent haematemesis, vomiting and fatigue. On physical examination, normal bowel sounds and a soft and a nontender abdomen were described. No alcohol, tobacco, or other drug use was declared. No endoscopical surveillance was performed. His serology showed slight anaemia (haemoglobin 11 g/dl and haematocrit 40%), without any other blood disorders.
The emergency upper gastrointestinal endoscopy which was done, revealed a superficial depressed tumour in the anastomotic area (type 0–IIc early gastric cancer). With the patient’s medical history as our basis, we decided to perform a surgical total gastrectomy of the remnant stomach and the oesophagogastric junction, with removal of the jejunal segment.
The gross examination of the surgical specimen revealed a 3 x 4 x 3 mm, superficial, depressed, early gastric cancer in the anastomotic area, on the lesser curvature, which was located at 5.5 cm from the oesophagogastric junction. On cut section, corresponding to and surrounding the tumour, haemorrhagic areas were observed in the gastric wall.
The histopathological examination of the surgical specimen revealed a tubulo–papillary gastric adenocarcinoma that invaded the lamina propria, the muscularis mucosae, and the submucosa, without lymph node metastases (pT1bN0 stage). The immunohistochemical profile revealed an agrressive tumour behaviour (p53>80% and HER–2 membranar positivity, Maspin negative) [Table/Fig-1]. Corresponding and adjacent to the tumour, a particular histopathological aspect was seen. Into the muscularis mucosae and the submucosa, abnormally large arteries with extremely thick walls, dilated arterialized veins, and vascular spaces with an indeterminate origin (artery or vein) with rich anastomosis were evidenced; some of them were thrombotic, and others were tortuous, being surrounded by haemorrhagic areas. Some of the vessels protruded through the muscularis mucosae, being linked to the mucosa. The peritumoral normal gastric mucosa also revealed abnormally enlarged vascular spaces [Table/Fig-1]. The muscularis propria and the subserosa also presented large arteries.
Histopathological findings of Dieulafoy’s lesion associated with early-stage gastric carcinoma. The adenocarcinoma involves the mucosa and muscularis mucosae (A) and presents HER-2 positivity (B). In the submucosal layer, vascular clusters containing enlarged thick-walled arteries can be observed (A-F). A large-persistent vessel is also seen within mucosa (E)
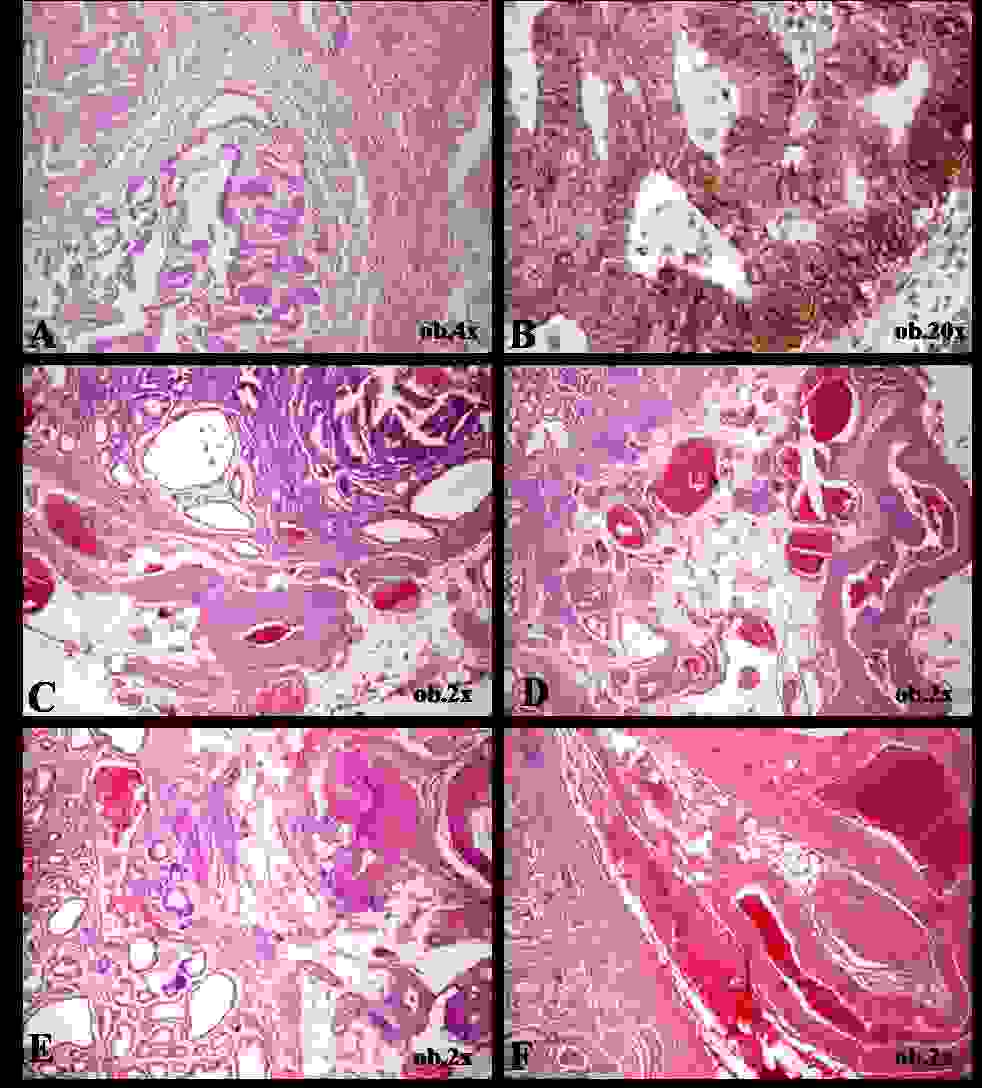
Based on the microscopic features which correlated with the recurrent haematemesis, the final diagnosis was an early GSC which was associated with Dieulafoy’s lesion. The postoperatory evolution was favourable; no adjuvant therapy was used. Our patient presented no complications six months after the surgery.
Discussion
Although gastric cancer presents a tendency to decline over time, it remains the second leading cause of the cancer-related deaths in the world, especially in the European countries, where most of the cases are diagnosed in the advanced stages. In contrast, in the Asian countries, a significant number of cases are diagnosed in the early stages, but the risk of the an associated–GSC following a remote gastric surgery seems to increase [7,8]. About 1–3% of the patients who undergo gastrectomies will present with cancer in the gastric remnant [8]. Despite the well–known risk, most of the remnant cancers are still diagnosed in the advanced stages, most of the early GSCs being reported in Japan [1,5,9].
Most of the studies revealed that GSCs occur after gastric surgeries which are done for both benign and malignant lesions, usually in elderly males who are aged about 65–70 years (M:F = 3:1), the interval between the initial gastrectomy and the GSC occurrence being longer in the cases that undergo surgery for benign diseases as compared to that in the malignant ones (30–32 vs 10–18 years) [7,9,10] and also that in the Billroth–II reconstruction being longer as compared to that in the Billroth–I reconstruction (30–32 vs 12–20 years) [9,10]. There are also anatomical differences with respect to the occurrence of GSC, it being usually located near the suture line and the remnant gastric wall in case of the Billroth–I reconstruction, respectively at the anastomotic area after a Billroth–II procedure [9,10]. Knowing these particularities is essential for a proper follow-up and for an early detection of a GSC; an efficient surveillance program supposes the annual endoscopic examination of the gastric remnant for about 10–12 years following a primary surgery, almost every second year after this period [9]. In the present case, in line with the literature data, a GSC occurred in a 75–year-old man, 38 years following a distal gastrectomy which was done for a peptic ulcer, with a Billroth–II reconstruction, in the anastomotic area. The early detection was not done to the proper surveillance, but rather to the associated haematemesis, as a result of the Dieulafoy’s lesion. However, this association seems to be incidental and no studies have been done with respect to a pathogenetic link among them.
The longer interval for the GSC occurrence in case of the benign lesions as compared to the malignant ones, could lead to a supposition that the surgical resection could be the favouring factor of the cancer, but the pathogenesis of a GSC following gastric cancer is more complex and it depends on the properties of the remnant mucosa [10]. Moreover, the Billroth–II procedure which was used in the past for the correction of the benign gastric lesions seems to favour the streaming of the gastric stump anastomosis and, consequently, the continuous bloating of the anastomotic area with bile acids [10,11], as important risk factors for the cancer genesis in this area.
In this case, the recurrent haematemesis was the main clinical symptom of the GSC. Recurrent or severe gastrointestinal bleeding was also the main symptom of the preoperative diagnosis in the other gastric tumours that were associated with the Dieulafoy’s lesion, as we have been shown in [Table/Fig-2]; three gastric carcinomas [1,4,5] and three stromal tumours [3,6] were reported to be associated with the Dieulafoy’s lesion, in the English literature [Table/Fig-2 and 3].
Search strategies performed on April 2, 2013, and results from Medline (via PubMed) and Cochrane Library regarding the topic of Dieulafoy’s lesion associated with other gastric tumors
| Database | Search terms | Results | Relevant findings |
|---|
| PubMed | Dieulafoy’s AND carcinoma OR Dieulafoy’s AND gastric carcinoma OR Dieulafoy’s AND GIST | 5 case reports | no reported cases about association with stump carcinoma |
| Cochrane Library | Dieulafoy’s AND carcinoma OR Dieulafoy’s AND GIST | 0 articles | no articles about the subject |
| Lilacs | Dieulafoy’s AND carcinoma OR Dieulafoy’s AND GIST | 0 articles | no articles about the subject |
| KoreaMed | Dieulafoy’s AND carcinoma OR Dieulafoy’s AND GIST | 0 articles | no articles about the subject |
The clinicopathologic features of the six PubMed-reported cases that revealed gastric tumors associated with Dieulafoy’s lesion (M = male; F = female; GIST = Gastrointestinal stromal tumor)
| Clinicopathologic features |
|---|
| Authors | Gender | Age (years) | Medical history | Symptoms | Endoscopy | Depth of invasion | Microscopic type |
|---|
| Vats et al., [10] | M | 86 | irrelevant | Melena | 4–cm submucosal tumor on the lesser curvature, with a protuberant shiny blood vessel | submucosa | Benign GIST |
| Seya et al., [11] | F | 64 | irrelevant | Massive hematemesis | 5–cm submucosal tumor on the lesser curvature of the lower gastric body, with a central Dieulafoy’s ulceration | submucosa | Benign GIST |
| Seya et al., [11] | M | 60 | irrelevant | Massive hematemesis | Gastric ulcer at first admission and 6–cm submucosal tumor on the greater curvature of the upper stomach, at the second endoscopy | submucosa | Benign GIST |
| Leone et al., [6] | M | 41 | irrelevant | Anemia Melena | 1.5–cm subcardial ulcerated lesion | mucosa | Signet-ring cell carcinoma |
| Kishikawa et al., [4] | M | 48 | irrelevant | Massive melena | Small ulcer with a large vessel protruding from the base and IIc early cancer on the posterior wall of the gastric fundus | muscularis mucosae | Poorly differentiated signet-ring cell adenocarcinoma |
| Taketsuka et al., [3] | M | 69 | irrelevant | Hematemesis Anemia | Dieulafoy’s ulcer and IIa early cancer in the upper body of the stomach | submucosa | Well differentiated adenocarcinoma |
| Present case | M | 75 | Distal gastrectomy for peptic ulcer 38 years ago | Hematemesis Anemia | IIc early cancer in the anastomotic area | submucosa | Well differentiated stump adenocarcinoma |
(M = male; F = female; GIST = Gastrointestinal stromal tumor)
Based on the particularities of the present case and the literature data, we conclude that a meticulous endoscopical surveillance with multiple biopsies should be the golden standard from the 10 to the 15th years after the initial surgery in both benign and malignant primary gastric lesions. Independent of the medical history, the Dieulafoy’s lesion and gastric cancer should be suspected in the patients with recurrent haematemesis and/or anaemia and even in normostenic patients with a good performance status.
(M = male; F = female; GIST = Gastrointestinal stromal tumor)
[1]. Kishikawa H, Nishida J, Hosoe N, Nakano M, Morishita T, Masamura S, Ando N, Terayama K, Ishii H, Gastric cancer associated with Dieulafoy’s lesion: case reportGastrointest Endosc 2003 57:969-72. [Google Scholar]
[2]. Dieulafoy G, Exulceratio simplex: l’intervention chirurgicale dans les hematemeses foudroyantes consecutives a l’exulceration simple de l’estomacBull Acad Med 1898 49:49-84. [Google Scholar]
[3]. Vats HS, Wengert TJ, Torbey CF, Gastrointestinal stromal tumor with Dieulafoy lesion: a novel associationClin Med Res 2006 4:228-29. [Google Scholar]
[4]. Leone O, Zanelli M, Santini D, Minni F, Marrano D, Dieulafoy’s disease associated with early gastric cancerJ Clin Pathol 1995 48:267-70. [Google Scholar]
[5]. Taketsuka S, Kasama K, Kakira Y, Horie K, Tagaya N, Kojima M, Maruyama K, Early gastric cancer located just above Dieulafoy’s ulcer, with massive bleedingGastric Cancer 2006 9:320-24. [Google Scholar]
[6]. Seya T, Tanaka N, Yokoi K, Shinji S, Oaki Y, Tajiri T, Life-threatening bleeding from gastrointestinal stromal tumor of the stomachJ Nippon Med Sch 2008 75:306-11. [Google Scholar]
[7]. Tokunaga M, Sano T, Ohyama S, Hiki N, Fukunaga T, Yamada K, Yamaguchi T, Clinicopathological characteristics and survival difference between gastric stump carcinoma and primary upper third gastric cancerJ Gastrointest Surg 2013 17:313-18. [Google Scholar]
[8]. Nozaki I, Nasu J, Kubo Y, Tanada M, Nishimura R, Kurita A, Risk factors for metachronous gastric cancer in the remnant stomach after early cancer surgeryWorld J Surg 2010 34:1548-54. [Google Scholar]
[9]. Komatsu S, Ichikawa D, Okamoto K, Ikoma D, Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y, Nakanishi M, Fujiwara H, Ochiai T, Kokuba Y, Otsuji E, Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomyWorld J Gastroenterol 2012 18:2832-36. [Google Scholar]
[10]. Tanigawa N, Nomura E, Lee SW, Kaminishi M, Sugiyama M, Aikou T, Masaki K, The Society for the Study of Postoperative Morbidity after Gastrectomy. Current state of gastric stump carcinoma in Japan: based on the results of a nationwide surveyWorld J Surg 2010 34:1540-47. [Google Scholar]
[11]. Fisher A, Graem N, Christiansen L, Causes and clinical significance of gastritis following Billroth II resection for duodenal ulcerBr J Surg 1983 70:321-25. [Google Scholar]