Hydatid disease, an endemic in the cattle grazing areas, particularly in the Asian region, is a parasitic infection which is caused by the cestode tapeworm, Echinoccocus granulosus. There is involvement of the intra–abdominal organs apart from the liver, in 10-15% of the cases. We are reporting a case, wherein the appendix was involved with this parasitosis and we have discussed the review of the literature regarding the diagnostic, surgical and the newer percutaneous approaches for the management of this disease.
Introduction
Humans may become infected with Echinococcus granulosus by eating contaminated food or by direct contact with infected animals. Human cystic echinococcosis, which is caused by E. granulosus, is the most common presentation and it probably accounts for more than 95% of the estimated 2-3 million annual worldwide cases [1,2].
Mass screenings have identified symptomatic and asymptomatic infections in patients, which have ranged in age from 6 years to the very elderly [2,3]. About 80% of the patients have symptoms, but the small (<5 cm in diameter) and uncomplicated cysts are usually asymptomatic [2,3,4]. The aim of this case report was to highlight the routes of dissemination, the diagnostic features and aids, and the treatment options of this rare clinical entity.
Case Report
A 30-years woman presented to the surgical outpatients department with the chief complaint of an off and on vague lower abdominal pain and discomfort which was confined to the right side, since the past 1 year. The patient had two episodes of low grade fever since the past month, which had lasted for 3-4 days and had subsided following an intake of anti-pyretics. She has no associated history of nausea, vomiting, diarrhoea, anorexia, fever, jaundice or significant weight loss. She was a daily wage worker in farms, a non-smoker and a non-alcoholic and she occasionally consumed a non-vegetarian diet. Her menstrual cycles were normal and her last period had occurred 15 days back with normal flow and it had lasted for 4 days. Her obstetric history was G4P3L3, with her last delivery having taken place 4 years back. There was no other significant past, medical or family history.
On physical examination, she was found to be thin built, with a weight of 64 kg and a height of 5 feet and 2 inches. Her systemic examination revealed her to be anicteric and afebrile, with a pulse rate of 104/min, a BP of 112/68 mmHg and an unremarkable cardio-pulmonary examination. On abdominal examination, no definite lump was found to be palpable, though mild tenderness was elicited in the right iliac and the hypogastric fossa. Her gynaecological examination revealed normal per vaginal findings. Her blood investigations revealed Hb of 9.5 gm%, a TLC of 8,400/cumm, a DLC of P–74, L–16, E–8, M–2, S. Bil (T)-1.1mg/dl, (D)– 0.7mg/dl and (I)-0.4 mg/dl. The abdominal ultrasound revealed a multi-loculated hypo-echoic mass which measured 10 x 12cm in size, which adhered to the right adenexal region, the caecum and the appendix. A presumptive diagnosis of a right ovarian cyst was offered and the patient was planned for a surgical removal of the abdominal mass. After exposing the abdominal cavity via a infraumblical midline incision, a cystic mass was observed, which overlay the small bowel loops in the right infraumblical region and adhered to the appendix, the mesoappendix and the caecum. No peritoneal tumour implants or ascites were conspicuous. The cyst was removed completely along with the appendix and it was sent for a histopathological examination.
Gross
The specimen comprised of a large cyst which was attached to the tip of the appendix [Table/Fig-1]. It measured 12 x 8 x 8 cm. The cut section of the cyst revealed cheesy material with three daughter cysts which measured 6 x 4 cm, 4 x 3 cm and 3 x 3 cm.
Cut section of Hydatid cyst with 3 daughter cysts and tip of appendix attached to serosal surface at 8 o’clock position

Microscopy
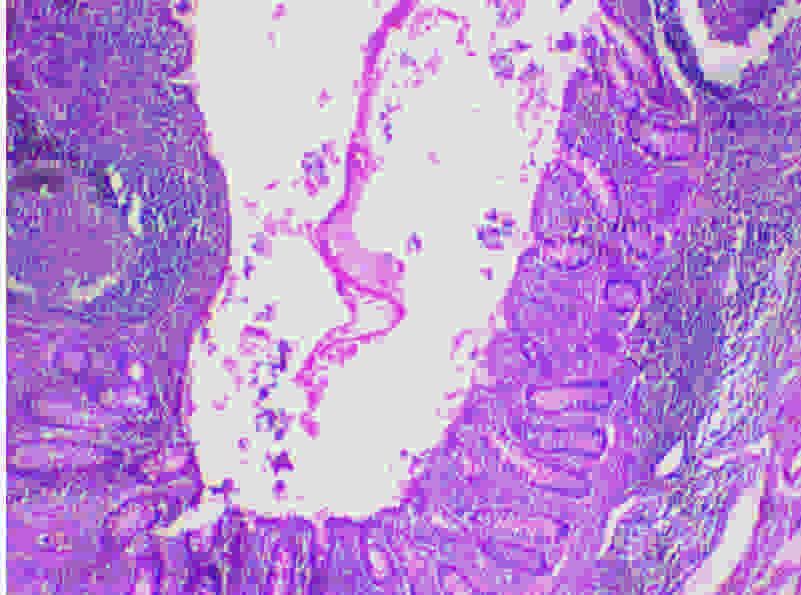
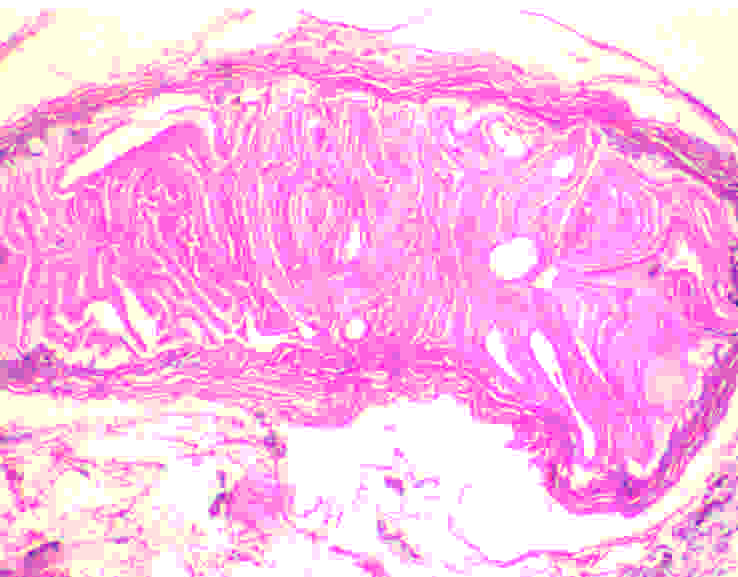
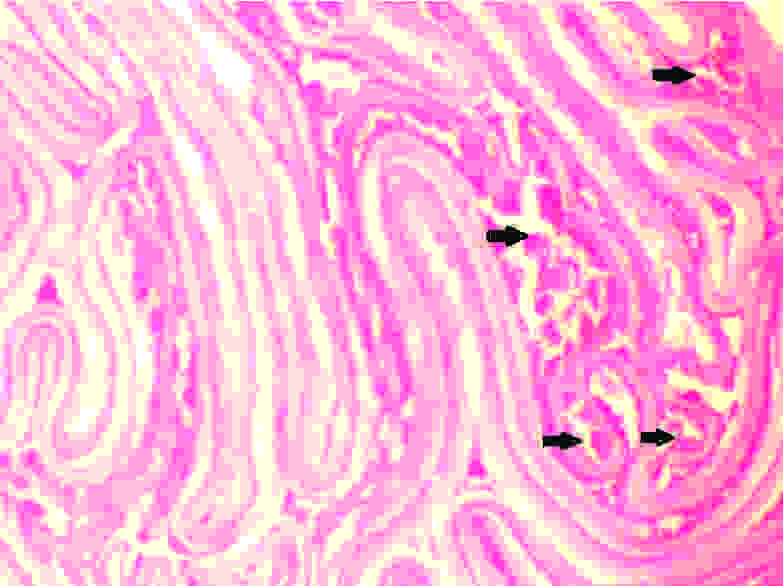
The histopathological examination revealed a luminal invasion of the appendix with a hydatid cyst wall [Table/Fig-2]. Sections from cyst wall showed an outer acellular laminated membrane along with an inner transparent germinal layer [Table/Fig-3]. On high power examination, calcified scolices were observed inside the germinal membrane [Table/Fig-4].
(H&E 40X): Lumen of appendix showing part of chitinous cyst wall
(H&E 40X): E. granulosus with a complex wall consisting of an outer acellular laminated membrane and an inner transparent germinal membrane
(H&E, 400X): Inner Germial layer with calcified scolices (arrow)
The post-operative course was uneventful and the patient was discharged on the 3rd post-operative day. A therapy with Albendazaole 400mg BD was administered for 4 weeks following the surgery. The patient was in good health and had no recurrence at 6 months of follow up.
Discussion
Hydatid disease is a common clinical pathology in many parts of the world. The disease has an endemic course in Asia and in certain parts of Europe [5]. In the endemic areas, the male-to-female ratio is approximately equal. Four species of the genus, echinococcus are known to cause infections in humans: echinococcus granulosus (cystic hydatid disease), echinococcus multilocularis (alveolar hydatid disease), echinococcus vogeli, and echinococcus oligarthus (both cause polycystic hydatid disease) [6,7].
Echinococcus granulosus requires two hosts. Dogs are the definitive hosts and the adult worms are found in their small intestines. Humans become accidental intermediate hosts either through contact with the definitive hosts or by consuming vegetables and water which are contaminated with the hydatid ova. Its eggs are ingested by the intermediate hosts like cows, sheep, and humans, which liberate embryos in the duodenum, which penetrate the intestinal mucosa and enter the portal circulation. The liver acts as a first filter, while the lungs act as second filters, which explains the frequent occurrence of the cysts in these locations. Only 10–15% of the embryos are free to develop cysts in other organs of the body, namely, the kidneys (2–4%), heart and pericardium (2–3%), bone (1–2%), spleen (1–2%), muscle (1%) and the brain (0.5–1%) [5,6].It has also been hypothesised that beside the portal circulation, the echinococcus embryos may spread via other routes like the lymphatic channels and the biliary tract or following the rupture of the primary cyst and dissemination into the peritoneal cavity, thus resulting in unusual presentations [7,8].
Clinically, the hydatid cyst enlarges progressively over a period of years and it presents with non specific signs and symptoms. The natural progression of an untreated cyst may include calcification and death of the cyst; however, more frequently, the cyst gradually enlarges. The major complication risk is the rupture of a hydatid cyst, which may trigger an anaphylactic shock. The other complications can include local infections, fistulas, haemorrhages, or compression of the adjacent tissues [6,9].
In the present case, the pressure effects on the surrounding tissues could have presented with ureteric, bladder or bowel obstructions or rarely, infertility. Infections or inflammatory reactions around the cyst cause irritation of the adjacent peritoneum, which could have mimicked acute appendicitis or salpingo-oophoritis. Our case presented with the similar symptoms of fever and lower abdominal pain following a secondary infection of the cyst. Due to the anatomical proximity and the symptoms, the clinical presentation was more towards that of a tubo–ovarian masss.
A hydatid cyst may be solitary or multiple. Sometimes, the cyst may resemble a multiloculated mass which fills the entire peritoneal cavity, which is referred to as peritoneal hydatidoses. For diagnostic purposes, ultrasonography (USG) is the first line of screening for abdominal hydatidosis, as it can detect the cystic membrane, the daughter cysts, the septa, and the hydatid sand [10]. CT scan best demonstrates the cyst wall calcification, infection and peritoneal seedling. The serological tests for the diagnosis of hydatid disease include immunoelectrophoresis (IE), enzyme – linked immunosorbent assay (ELISA), and the indirect haemagglutination test (IHA) [11].
Surgical excision is the mainstay of the treatment for hydatid cysts of the liver. Resection of involved organ and pericystectomy are the procedures that resect the closed cysts with a wide safety margin; however, some consider this procedure as too radical, for hydatid cyst removal. The new therapeutic approaches include percutaneous punction of the cyst and aspiration of the internal fluid, followed by injection and re–aspiration of alcohol containing solutions. This so–called PAIR procedure (Puncture Aspiration Injection Re–aspiration) is repeated several times and it has been advocated as a safe and a simple approach for treating hydatid cysts. A trial which was conducted by Yahya et al., in 109 patients, demonstated the successful results of the percutanoeus administration of a 10% albendazole solution and a 20% hypertonic saline solution in the cystic cavities under sonographic guidance [12]. Antihelminthic drugs are administered for prolonged periods in the post-operative phase, to avoid a recurrence which is secondary to a peritoneal spillage during the resection. Recurrence is defined as the appearance of new active cysts after the therapy, which include a reappearance with a continuous growth of live cysts at the site of a previously treated cyst or the appearance of a new distant disease which results from spillage. The overall local recurrence rate of hepatic hydatid disease has been reported to be approximately 10–12% and so the patient has to be kept on a regular follow up to avoid these late complications [13,14].
Conclusion
An extrahepatic hydatid cyst is rare and it should be included in the differential diagnosis of any intra-abdominal cystic lesion, especially in the regions where the hydatid disease is endemic. It is preferable not to biopsy or to aspirate the lesion, if one suspects the lesion to be a hydatid cyst. An en-bloc resection without rupture and consequent spreading of daughter cysts is the recommended treatment strategy and it has been accepted as curative, which can avoid further recurrence.
[1]. Krasniqi A, Limani D, Gashi-Luci L, Spahija G, Primary hydatid cyst of the gallbladder: a case reportJournal of Medical Case Reports 2010 4:29 [Google Scholar]
[2]. Sayek I, Tirnaksiz MB, Dogan R, Cystic hydatid disease: current trends in diagnosis and managementSurg Today 2004 34:987-96. [Google Scholar]
[3]. Parray FQ, Wani SN, Bazaz S, Khan SR, Primary Pelvic Hydatid Cyst: A Case Reportdoi:10.1155/2011/809387 [Google Scholar]
[4]. Fazili A, Wani NA, Khan TS, Mir AR, Hydatidosis: Rare PresentationsJK-Practitioner 2002 9(4):252-53. [Google Scholar]
[5]. Nadeem N, Khan H, Saulat Fatimi S, Ahmad MN, Giant Multiple Intra-Abdominal Hydatid CystsJ Ayub Med Coll Abbottabad 2006 18(4):70-72. [Google Scholar]
[6]. Ezer A, Nursal TF, Noyan T, Moray G, Giant hepatic hydatid cyst: A case reportWorld J Gastroenterol Apr 2007 13(16):2388-89. [Google Scholar]
[7]. Daali M, Hssaida R, Peritoneal hydatidosis: a study of 25 cases in MoroccoSante 2000 7-8(10(4)):255-60. [Google Scholar]
[8]. Gollackner B, Langle F, Radical surgical therapy of abdominal cystic hydatid disease: factors of recurrenceWorld J Surg 2000 June 24(6):717-21. [Google Scholar]
[9]. Balik AA, Celebi F, Basglu M, Oren D, Yildirgan I, Atamanalp SS, Intra-abdominal extrahepatic echinococcosisSurg Today 2001 10:881-4. [Google Scholar]
[10]. Akhan O, Ozmen MN, Dincer A, Gocmen A, Kalyoncu F, Percutaneous treatment of pulmonary hydatid cystsCardiovasc. Intervent. Radiol 2004 17:271-75. [Google Scholar]
[11]. Prousalidis J, Kosmidis C, Anthimidis G, Kapoutzis K, Postoperative recurrence of cystic hydatidosisCan J Surg 2012 Feb 55(1):15-20. [Google Scholar]
[12]. Paksoy Y, Odev K, Sahin M, Arslan A, Koc O, Percutaneous treatment of hepatic hydatid cysts: comparison of direct injection of albendazole and hypertonic saline solutionAmerican journal of roentgenology 2005 185(3):727-34. [Google Scholar]
[13]. Parray FQ, Gagloo MA, Bhat AH, Chowdri NA, Noor MM, Peritoneal hydatidosisThe Internet Journal of Surgery 2007 9(2) [Google Scholar]
[14]. Sielaff TD, Taylor B, Langer B, Recurrence of hydatid diseaseWorld J Surg 2001 Jan 25(1):83-86. [Google Scholar]