Appendiceal Carcinoma with Krukenberg’s Tumour Mimicking Primary Ovarian Cancer
Clement Wilfred D.1, Vijaya Mysorekar V.2, Sulatha Kamath3, Saurabh Singla4
1 Associate Professor, Department of Pathology, MSRMC, Karnataka, India.
2 Senior Professor, Department of Pathology, MSRMC, Karnataka, India.
3 Professor, Department of Pathology, MSRMC, Karnataka, India.
4 Saurabh Singla, Assistant Professor, Department of Radiology, MSRMC, Karnataka, India.
NAME, ADRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Clement Wilfred D., No. 76 A, First Main Road, John Bull Street, Viveknagar Post, Bangalore-560047, Karnataka, India.
Phone: 9945226314,
E-mail: clement.wilfred@yahoo.com
Appendiceal adenocarcinoma (AACa) is a rare tumour which represents 0.5% of all gastrointestinal malignancies. The prognosis is poor, because it is usually found at an advanced stage, that in turn, is partly due to a low threshold of suspicion and difficulties in diagnosis prior to surgery. It may occasionally demonstrate ovarian metastases that are large and which dominate the clinical and radiological presentations, leading to a misdiagnosis of an ovarian primary malignancy. We are reporting a case of an occult AACa which manifested clinically as a primary ovarian cancer which was at an advanced stage. Staging laparatomy revealed large bilateral ovarian tumours of clinical FIGO Stage III, with presumed appendiceal implants. Histological examination revealed a mucinous adenocarcinoma with a signet ring component, which involved bilateral ovaries and the appendix transmurally. Immunophenotypic analysis revealed a positive expression of CK 20 and CDX 2 and absence of CK 7 staining, which was compatible with appendiceal primary and ovarian metastases. The diagnosis was subsequently revised to AACa with Krukenberg’s metastasis, Stage IV. Although AACas are uncommon, they should be considered in the differential diagnosis of intraabdominal masses and the distinction between ovarian and appendiceal primary malignancies is critical, as the treatment modalities vary.
Appendiceal malignancy, Krukenberg’s tumour, Ovarian metastasis, Ovarian carcinoma
Case Report
A 54-years old postmenopausal woman, P4 L4, presented with pain and a mass per abdomen, of two months duration. Abdominal examination revealed a single, hard, lobulated, central pelvic mass which measured 16x15 cm. Abdominopelvic ultrasonography and contrast enhanced computed tomography revealed heterogeneous, large, bilateral, solid and cystic adnexal masses (? malignant) with moderate ascitis [Table/Fig-1]. No obvious appendiceal mass was evident.
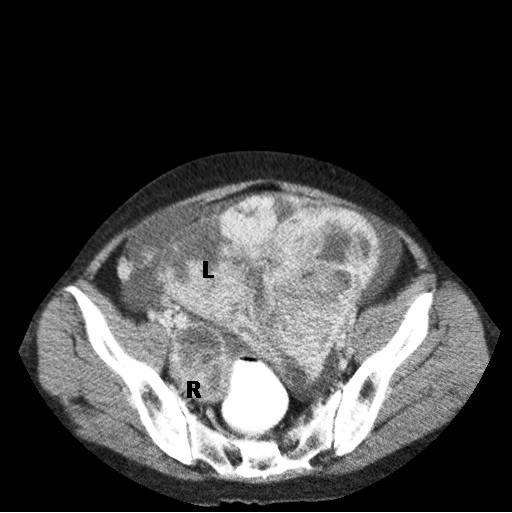
Ascitic fluid examination revealed malignant cells and blood tests showed elevated CA125 levels (51.5 U/ml). A complete digestive tract endoscopy which was done was found to be normal and gastric and colonic biopsies which were done were normal.
Based on the clinical presentation, physical examination and tumour marker and radiographic studies, a clinical diagnosis of an ovarian malignancy was made and a staging laparatomy was performed, which revealed bilateral large ovarian tumours, the intraoperative frozen sections of which revealed a malignancy. Macroscopic greater omental, uterine serosal and “presumed” appendiceal serosal implants were present and a clinical FIGO Stage III was assigned. Total abdominal hysterectomy, bilateral salpingoophorectomy, omentectomy and appendicetomy were performed.
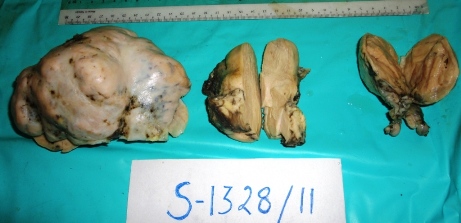
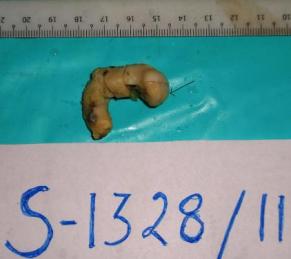
On macroscopy, the right and left ovarian tumours were found to be coarsely lobulated and predominantly solid, with mucoid cystic areas, a heterogeneous yellowish white cut surface, an intact cerebriform capsule and which measured 8.5x6x5 cm and 16x 13x9.5 cm respectively [Table/Fig-2]. The appendix weighed 65 g , it measured 5cm long and 1 to 2.5cm in diameter, with a nodular enlargement of the distal third, that on sectioning, revealed a grey white, ill circumscribed mass which measured 2.5x2x1.5 cm, which exhibited mucoid areas [Table/Fig-3].
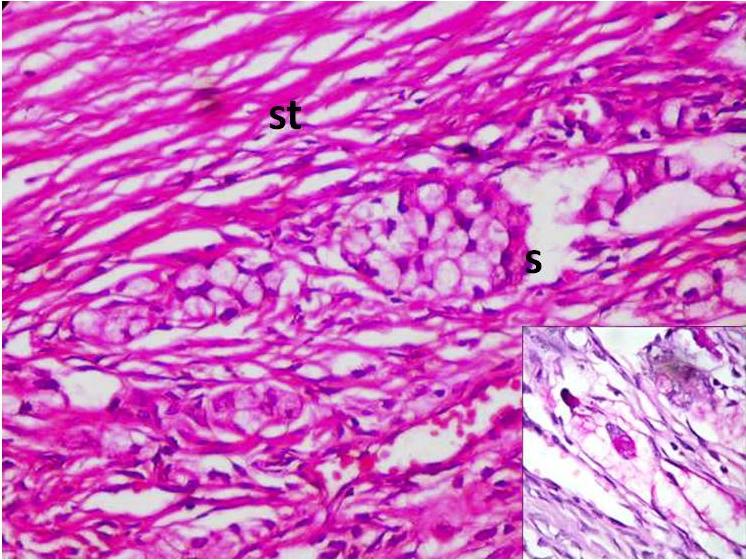
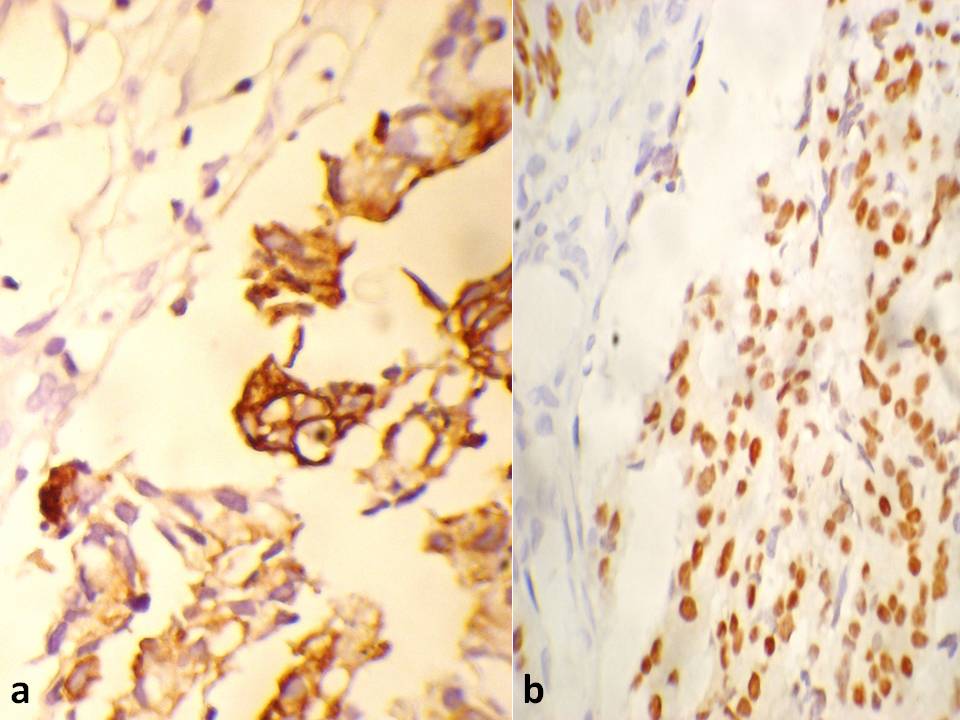
A histological analysis revealed a coexistent i) transmurally infiltrating appendiceal mucinous ACa, Grade 3 [Table/Fig-4] with a focal, mucin rich, PAS positive signet ring component [Table/Fig-5] and a mesoappendiceal invasion and ii) a bilateral mucinous ovarian ACa, Grade 3 [Table/Fig-6] with a focal, mucin rich, PAS positive signet ring component, which comprised 20% of the tumour [Table/Fig-7], along with multiple greater omental and uterine serosal invasive implants. Immunohistochemical staining which was done to elucidate the origin and character of the tumour cells revealed positive expressions of CK 20 and CDX 2 and absence of staining for CK 7 in the appendiceal and ovarian tumours [Table/Fig- 8]. These histological and immunohistochemical results allowed us to make a diagnosis of a primary AACa with a bilateral Krukenberg metastasis and a peritoneal dissemination; pT4G3 pNx pM1; TNM Stage IV.
Transmurally infiltrating appendiceal mucinous ACa, Grade 3
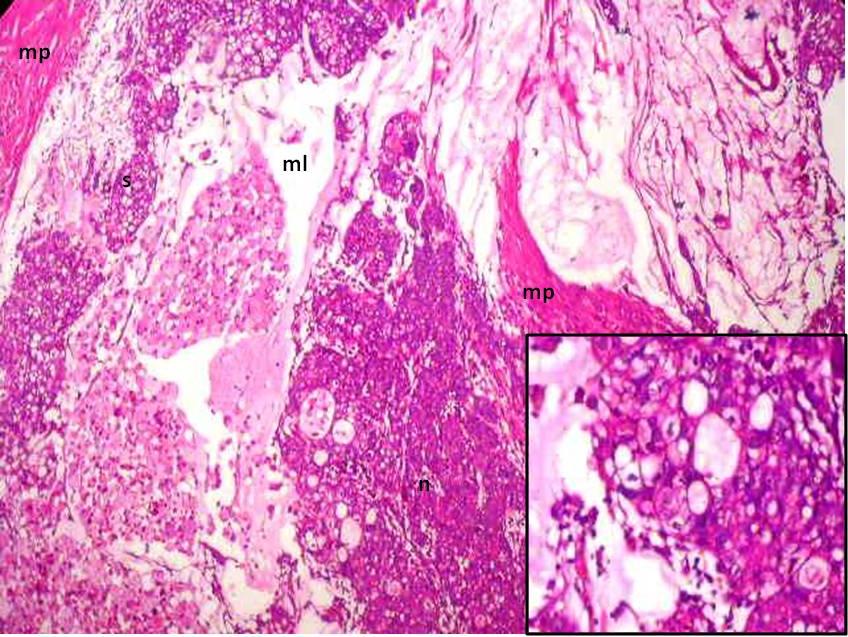
PAS positive signet ring component
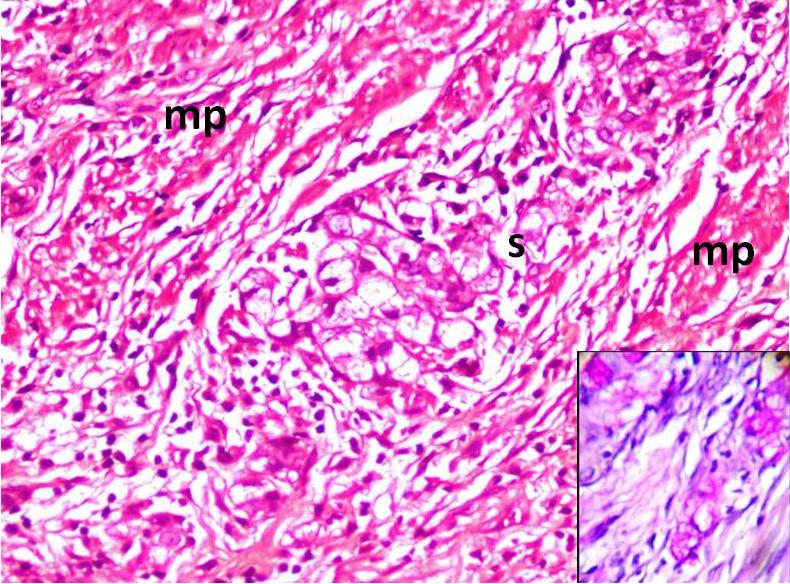
A bilateral mucinous ovarian ACa, Grade 3
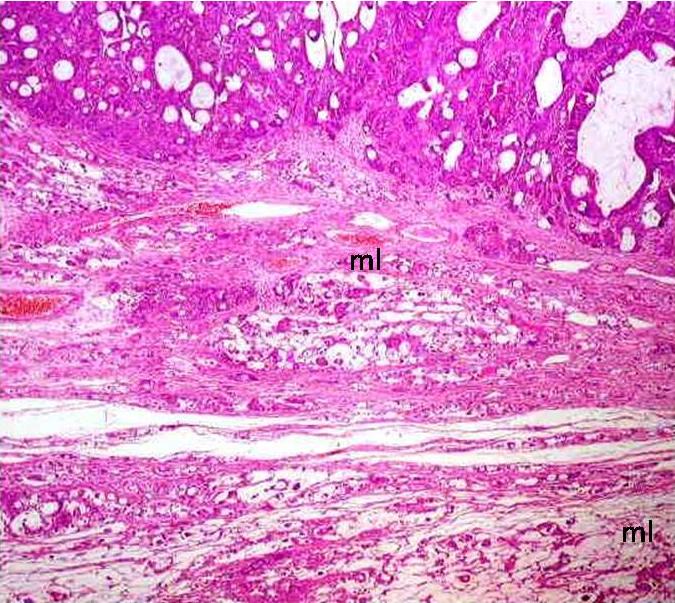
PAS positive signet ring component, which comprised 20% of the tumour
Absence of staining for CK 7 in the appendiceal and ovarian tumours
Discussion
Appendiceal malignancies are rare and they are diagnosed in only 0.9 to 1.4 % of appendicectomy specimens [1]. Epithelial tumours form a majority of these malignancies and they exhibit diverse histologies, which are comprised of i) carcinoids (85%) ii) ACas and iii) adenocarcinoids (2%). The ACas exhibit four morphological patterns i) mucinous ii) colorectal iii) mixed mucinous and signet ring and iv) signet ring type [2]. Mucinous AACa accounts for 5% of appendiceal cancers, with an average age of 58 years at diagnosis, with an even sex distribution and an overall 5 year survival of 46% [2]. The presence of signet ring cells is an independent prognostic indicator of a poor survival [3].
Ovarian metastases are encountered in 16.7 to 37 % of AACas [1].Ovarian metastases from mucinous AACas mimic Primary Ovarian Mucinous Carcinomas (POMCs) and their distinction is challenging. A transmural involvement with a mesoappendiceal spread, which was evident in the present case, was indicative of an appendiceal primary malignancy, unlike a metastasis, which would have demonstrated a preponderant serosal involvement.
Features that favour a metastatic mucinous ovarian carcinoma are i) bilaterality ii) a multinodular surface iii) an irregular infiltrative growth iv) single cell invasion v) angiolymphatic invasion and vi) an extraovarian spread [4]. In the current case, the presence of features i), ii), iii), iv) and vi) were indicative of an ovarian secondary malignancy, but POMCs can be bilateral (5%), they can exhibit an extraovarian spread and further, POMCs of the” infiltrative” type can exhibit features iii) and iv) [4]. Even though the bilateral ovarian tumours in the present case had focal signet ring components with desmoplastic/cellular stromal responses, which qualified for a diagnosis of Krukenberg’s tumour, the possibility of them being POMCs with a signet ring cell differentiation, which have been rarely described in literature, existed [5].
Thus, only on the basis of a conventional pathological examination, it was difficult to establish unequivocally, whether the carcinoma had originated from the ovary or the appendix. Hence, we used immunohistochemistry for cytokeratins and CDX2.
The coordinate expression of CK7 and CK20 is useful. The ovarian carcinomas are CK7 + / CK20 – and the AACas are CK7– / CK20 +. CDX2 is a sensitive marker for colorectal carcinoma which metastasizes to the ovary.
The differential diagnosis is clinically important, since the therapeutic approach is totally different for ovarian and appendiceal cancers. An optimal surgical debulking is advocated for ovarian tumours and chemotherapy with Taxol and carboplastin is effective, whereas the clinical utility of radical tumour debulking and chemotherapy remains unknown for appendiceal cancers. [1,6] However, an aggressive cytoreductive surgery and a perioperative intraperitoneal chemotherapy with Mitomycin-C and 5-fluorouracil could be used for appendiceal cancers with peritoneal disseminations [1].
In conclusion, metastatic AACas should be considered in the differential diagnosis of mucinous ovarian tumours with signet ring cells, especially when these tumours are associated with extraovarian disease and are bilateral. The appendix should always be carefully examined during explorations for ovarian masses and prophylactic appendicectomies should be considered as a part of the treatment of ovarian carcinomas. Further, without a meticulous clinicopathological correlation, the potential for a misdiagnosis of pelvic tumours remains high.
[1]. Jun S, Shinsuke K, Jon K, Hiroshi U, Tetsuro M, Hirokazu N, Signet Ring Cell Carcinoma of the Appendix Manifesting as Colonic Obstruction and Ovarian tumors: Report of a CaseSurg Today 2009 39:235-40. [Google Scholar]
[2]. Carl R, Louay H, Vladimir G, Ghulamullah S, Muhammad WS, Cancers of the Appendix: Review of LiteratureISRN Oncologyvol. 2011Article ID 728579, 6 pages, 2011. doi:10.5402/2011/728579 [Google Scholar]
[3]. Valentina RB, Federico C, Antonio DP, Peritoneal seeding from appendiceal carcinoma: A case report and review of the literatureWorld J Gastrointest Surg 2010 2(8):265-69. [Google Scholar]
[4]. Jaime Prat, Ovarian carcinomas, including secondary tumors: diagnostically challenging areasModern Pathology 2005 18:S99-S111. [Google Scholar]
[5]. Samer ES, Ulrich S, Hans RT, Andreas H, Karsten M, Primary Signet Ring Cell Mucinous Ovarian Carcinoma: A Case Report and Literature ReviewCase Rep Oncol 2010 3(3):451-57. [Google Scholar]
[6]. Gehrig PA, Boggess JE, Ollila DW, Groben PA, Van LL, Appendix cancer mimicking ovarian cancerInt J Gynecol Cancer 2002 12:768-72. [Google Scholar]