The study was conducted on thirty female healthy adult wistar albino rats. The wistar albino rats (220 ± 20g) with regular 4-day estrus cycles were procured from Kings Institute, Guindy, Chennai, India. The rats had free access to standard rat pellet with water supplied ad libitum and strict hygienic conditions. Rats were habituated to laboratory conditions (i.e. room temperature of 25 ± 2°C; relative humidity 45% to 55% and a 12:12 light/dark cycle) for 48 hours prior to experimental protocol to minimise non–specific stress if any. The approval of the Institutional Animal Ethical Committee (IAEC) of Saveetha University (IAEC No.Anat.002/2009) was taken prior to the experiments. All the protocols and the experiments were conducted in strict compliance according to ethical principles and guidelines provided by Committee for the Purchase of Control and Supervision of Experiments on Animals (CPCSEA).
The experiment was carried out for a period of 2–4 months on sample of with 30 healthy female adult wistar albino rats (age 2 months).
A single longitudinal skin incision was made on the dorso-lateral area at the level of the lower poles of the kidney after shaving the furs with scissors and cleansing with 70% alcohol. The skin was retracted laterally toward one side and the ovary exposed through a thin muscle mass just below the dorsal muscle mass. Each incision was of minimum length to allow the ovary extrusion. Ligation of the upper horn of the uterus, including the arteries with chromic catgut was carried out. Ovary was excised and wounds were closed. High degree of aseptic procedure was maintained throughout the operation. Operations were carried out in estrus phases as determined by vaginal smears using PAP smear. Five groups of female rats (n=6) were included in this study. During this period, the rats were given ethanol and FP. Powder FP was prepared from seeds of P. corylifolia and delivered to the rat via gavage technique. Both water ad lititum and ethanol were kept in cage with free access method. Number of days each animal will be housed: 2–4 months. At the end of 4 months, the animals were anaesthetised with ether and intra–cardiac perfusion of normal saline followed by 10% formal saline was done.
The brains of both the groups were dissected out, fixed in Golgi-Cox solution. After fixation, the brains were embedded in paraffin. After that, 35 micron coronal sections were done from embedded paraffin at the level of hippocampus. It was stained with toluidine blue to find out qualitative histological changes following ethanol induced FP. The first six neurons encountered in each animal when moving from medial to lateral in the sections were selected for quantitative analysis. Camera Lucida drawings of dendritic arborisation were made at a magnification of 400x. According to method of Sholl 1953, concentric circles were superimposed over the drawings of dendritic tree with increasing radius equal to geometric equivalent of 20 micron at a magnification of 400x, so that cell body was placed in the centre of the innermost circle[5]. The number of dendrites intersecting each circle was counted and compared group wise.
Methods Adopted
Diameter of cells
The diameter of the cells of the CA1, the CA3 region of the hippocampus were calculated. The diameter of the cell was calculated using the formula:

The maximum length, maximum breadth of cells was calculated using ocular micrometer. Then, the diameter of the cells of CA3 of hippocampus was calculated for all animals (n=6) in a group and the results are tabulated.
Total number of cells in the square
The cresyl violet stained sections of CA region of the hippocampus were focused under 40x objective lens of the microscope. As calibration constant differs for each microscope, for each objective same microscope with same objective, same magnification was used. Counting of neurons was done under 400x magnifications.The pyramidal cells of CA3 emerging from the mouth of dentate gyrus and the pyramidal cells of CA1, which is a continuation of CA3 was counted. The cell region for calculation was selected using random selection technique from the serial sections made for each group.
Packing density of cells
The packing density was calculated by the following formula
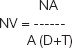
Where,
NV – numerical density or number of cells per cubic mm
NA - Average number of cells in the ------- per sq mm.
A - Area of reticule in sq mm
T - Thickness of the section in mm
D - Mean diameter of the cells in mm
A - Area of Reticule In Square Millimeter
Area of reticule = 380 x 380 Sq. Microns
= 144400 Sq. Microns
= 0. 144400 Sq.mm
T - Thickness of the section in millimeter
T = 4 Microns
= 0.004 mm
Histomorphometric Studies Result
Mean ± SEM of diameter of neurons (μ) in FP extract and/or ethanol treatment in rats
| Group | CA1 | CA2/3 | CA4 | Dentate gyrus |
|---|
| Group I | 3.55 ± 0.14 | 3.15 ± 0.15 | 1.90 ± 0.12 | 3.2 ± 0.18 |
| Group II | 2.19 ± 0.14 | 2.35 ± 0.16 | 1.84 ± 0.18 | 2.88 ± 0.27 |
| Group III | 3.33 ± 0.12 | 3.75 ± 0.25 | 1.98 ± 0.14 | 3.92 ± 0.16 |
| Group IV | 2.09 ± 0.14 | 2.15 ± 0.16 | 1.52 ± 0.18 | 2.68 ± 0.27 |
| Group V | 2.54 ± 0.14 | 2.45 ± 0.14 | 1.88 ± 0.2 | 2.94 ± 0.29 |
Means with different different group in each row are significantly different (p<0.05).
Mean ± SEM of total number of neurons in FP extract and/or ethanol treatment in rats
| Group | CA1 | CA2/3 | CA4 | Dentate gyrus |
|---|
| Group I | 202.9 ± 14.2 | 251.7 ± 5.7 | 168 ± 9.5 | 928.6 ± 24.5 |
| Group II | 172.5 ± 12.2 | 196.9 ± 9.3 | 140.8 ± 5.5 | 689.5 ± 14.5 |
| Group III | 212.7 ± 10.2 | 241.2 ± 6.8 | 160 ± 7.5 | 919.5 ± 15.6 |
| Group IV | 162.5 ± 8.2 | 176.9 ± 8.3 | 110.8 ± 7.5 | 589.5 ± 18.5 |
| Group V | 197.3 ± 16.2 | 211.2 ± 8.3 | 134.2 ± 6.5 | 710.5 ± 17.2 |
Means with different groups in each row are significantly different (p<0.05).
Mean ± SEM of packing density (X103/cubic mm) of neurons in FP extract and/or ethanol treatment in rats
| Group | CA1 | CA2/3 | CA4 | Dentate gyrus |
|---|
| Group I | 141.83 ± 7.52 | 143.63 ± 11.1 | 118.42 ± 5.62 | 234.16 ± 6.50 |
| Group II | 107.17 ± 3.55 | 113.83 ± 4.25 | 102.5 ± 7.2 | 190.4 ± 8.58 |
| Group III | 136.33 ± 6.74 | 134.33 ± 7.87 | 114.1 ± 6.84 | 232.18 ± 5.81 |
| Group IV | 97.17 ± 5.55 | 103.83 ± 6.27 | 92.61 ± 7.2 | 180.22 ± 4.58 |
| Group V | 116.6 ± 7.79 | 120.17 ± 3.07 | 98.35 ± 8.12 | 198.46 ± 8.65 |
Number of cells in per unit (cubic mm) were determined
Discussion
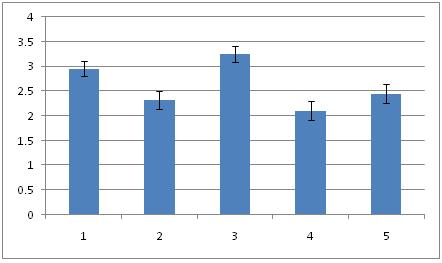
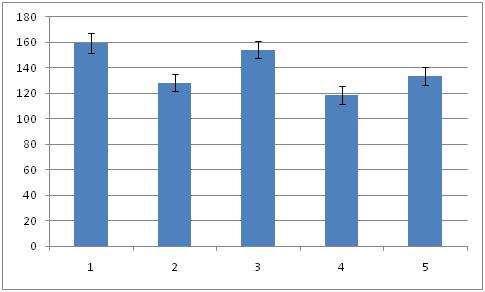
Histomorphometric investigations carried out on hippocampus proper and dentate gyrus of all the groups. The overall mean diameter, number and numerical density of the neurons were calculated by using in [Table/Fig-1,2,3,4,5and6]. Overall ratio was increased in Gr I [Table/Fig-7] (2.95 ± 0.15; 299.93 ± 13.48; 159.51 ± 7.69) as compared to Gr II [Table/Fig-8] (2.32 ± 0.19; 234.93 ± 10.38; 128.48 ±6.77). This might be the effect of estradiol action on hippocampus. In group III, diameter was increased [Table/Fig-9] (3.75 ± 0.17) than control groups. It may be due to the effect of FP. But the number and density of neurons was more than (658.35 ± 10.03; 154.24 ± 6.87) to [Table/Fig-7] 299.93 ± 13.48; 159.51 ± 7.69). The reason may be extragonadal action of estrogen. Thickness of the pyramidal cell layer and the numerical density of its neurons were significantly lower in group IV (2.11 ± 0.19; 118.46 ± 7.15) than in [Table/Fig-8] (2.32 ± 0.19; 128.48 ± 6.77). Mitra et al., 1999 investigated the density of neuron in alcoholic[6]. There was significant reduction (p<0.001) in density neuron per sq.mm section in CA-1, CA-2, CA-3 areas of hippocampus in rat fed with 10% ethanol for 6 months. There was also a decay in the total number of pyramidal neurons in [Table/Fig-10] (197.75 ± 10.63). But in [Table/Fig-10], the diameter, number and packing density were reduced compared to [Table/Fig-9].
Diameter of neurons (Mean ± SEM) in the hippocampal formation of all the groups. Fructus psoralea treated group significantly different from other group.
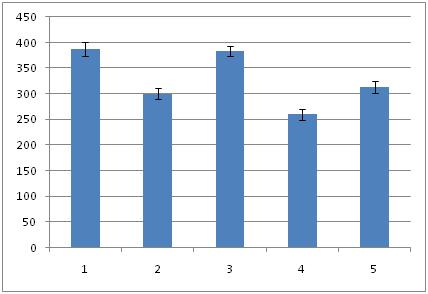
Total number of neurons (Mean ± SEM) in the hippocampal formation of all the groups. Fructus psoralea treated group significantly different from other group.
Packing density of neurons (Mean ± SEM) in the hippocampal formation of the all the groups. Fructus psoralea treated group significantly different from other group.
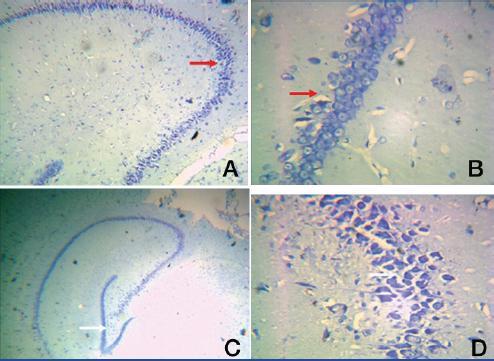
Group I: A. A photomic of a section of CA1/CA3 -Toludine blue -100X
B. A photomic of a section of CA1/CA3 – Toludine blue-400X
C. A photomic of a section of DG evenly distributed dentate cells (red arrow) – Toludine blue -100X
D. A photomic of a section of DG –presence of granule and pyramidal cells. Toludine blue-400X
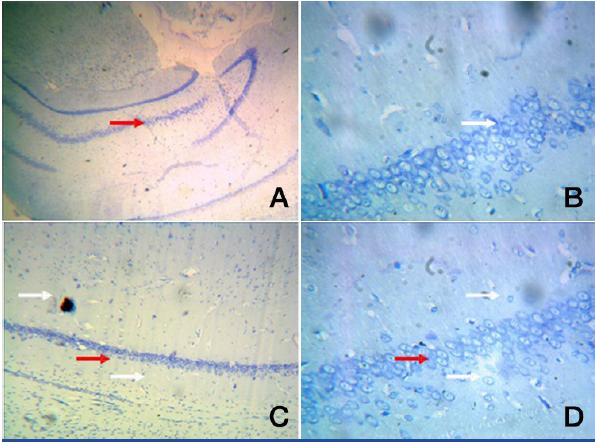
Group II: A. Photomic.of a section of CA1/CA3 - toludine stain X40
B. Photomic. of a section of CA1/CA3 – toludine stain X400
C. A Photomic. of a section of DG – toludine stain X100
D. A Photomic. of a section of DG - toludine stain X400
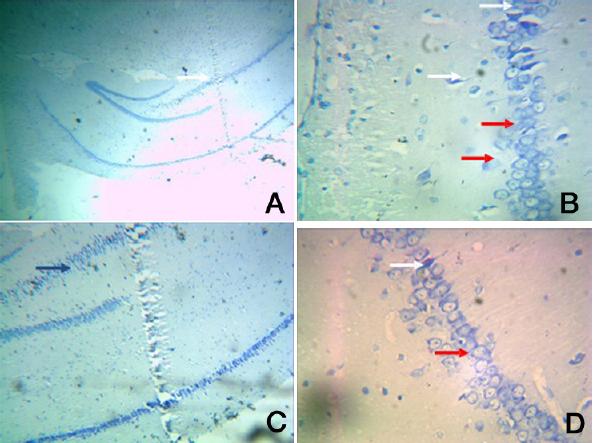
Group III: A. Photomic.of a section of CA1/CA3 – Toludine blue stain X100
B. Photomic of a section of CA1/CA3 – Toludine blue stain X 400.
C. Photomic.of a section of DG – Toludine blue stain X 100
D. Photomic.of a section of DG – Toludine blue stain X 400
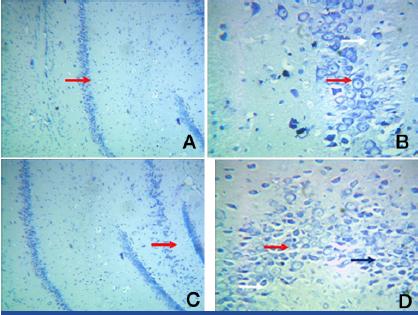
Group IV: A. Photomic of a section of CA1/CA3 Toluidine blue X100
B. Photomic of a section of CA1/CA3–Toluidine blue X400
C. Photomic of a section of DG–Toluidine blue X100
D. Photomic of a section of DG–Toluidine blue X400
There is also data to suggest that ethanol alters spine density of hippocampus [7,8], a brain region critical for the formation of some types of memories [9,10].
In the present study, diameter, density and number of neurons were increased [Table/Fig-9] (3.25 ± 3.25; 383.35 ± 10.03; 154.24 ± 7.17) and [Table/Fig-11] (2.45 ± 0.19; 313.3 ± 12.05; 133.39 ± 6.91) mainly in the sub–granular zone of dentate gyrus which was histologically unearthed by the present study. It may be due to the development of fresh neurons. The recent discovery that the hippocampus was able to generate new neurons (i.e., neurogenesis) throughout the lifespan of mammals, including humans. It had long been accepted that fresh dendritic spines or branches could form new synapses and thereby reinforce associations between cells or establish new ones. This capacity for connectivity was one among several means by which spines could participate in the formation of new associative memories[11]. O’Malley et al., reported a transient increase in spine density in the dentate gyrus improves spatial learning[12]. Archana et al., revealed that FP showed better ethanol tolerance and might be more effective in preventing neuronal death[13]. The subgranular zone of the dentate gyrus (SGZ) contains neural stem/progenitor cells (NS/PCs) capable of producing thousands of new granule cells per day [14]. These newborn hippocampal neurons are functionally integrated with existing neuroanatomical circuit[15] and are positively correlated with hippocampus-dependent learning and memory processes[16] . Dendritic spines are potential source of improving contact between excitatory neurons thereby improving cognition[17–19].
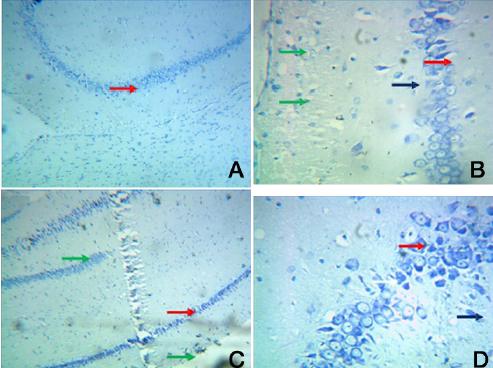
Group V: A. Photomic of a section of CA1/CA3–Toludine blue X100
B. Photomic of a section of CA1/CA3–Toludine blueX 400
C. Photomic. of a section of DG–Toludine blue X40
D. Photomic. of a section of DG–Toludine blue X400
[Table/Fig-8] showing the dentate gyrus granule cell layer with few mature granule cells and increased number of necrotic cells(white arrow). Some cells appeared with pale stained cytoplasm–(red arrow). Disorganised cells with sparse nuclei were clearly visible (white arrow) [Table/Fig-8B & 8D].
Toluidine blue impregnation reveals abundant CA1 & CA3 neurons. The neurons were densly packed in DG. Number of CA1 & CA3 hippocampal neurons were increased. The presence of astrocytes like cells (small triangular with round nuclei) around the large pyramidal nuerons (white arrow) was observed.
Toluidine blue stained sections revealed thin dentate gyrus granule cell layer that contained numerous apoptotic cells (green arrow) with darkly stained more number of apoptopic cells surrounded by numerous microglia (blue arrow) [Table/Fig-10D]. The hilus showed granule cells and pyramidal cells arranged in distorted manner (white arrow).
In [Table/Fig-11C] more number of immature neurons in the hilar region of sungranular DG were observed (White arrow).
Conclusion
The present study shows that number, denstiy and diameter of neurons were increased in group III & group V. Thus, presence of more number of neurons shows that there may be a migration of progenital neural stem cells from the ependymal layer of subventricular zone. The results suggest that administration of FP had induced neurogenesis of hippocampus which suggest that FP could serve in stabilizing brain morphology and physiology.
In present study, herb Fructus Psoralea is more efficient in producing neuroprotective effects in ethanol induced neurodegeneration of hippocampus of female Wistar albino rats. FP protects especially the more sensitive cells of CA1 & CA 3 region of hippocampus increasing their packed cell density. Finally, in both the regions, FP is a herb of choice to treat illness in CA region. As ethanol affects most of the neurons in cornu ammonis region of hippocampus, the herb FP may be used as a supplement to alleviate the harmful effects of ethanol, thereby improving the histomorphometric activity and memory.
Hippocampus is one of the first regions in brain to suffer from Alzheimer’s disease, encephalitis, medial temporal lobe epilepsy. People with extensive hippocampal damage may experience amnesia, learning and related memory disabilities. Hence the herb FP may be used as an adjuvant to treat the above neurological disorders efficiently with fairly good outcomes.