Histopathological Changes in the Liver of Rabbits Exposed to High Nitrate Ingestion in Drinking Water
Manoj Kumar Sharma1, Hemlata Sharma2, Neelam Bapna3
1 Associate Professor, Department of Anatomy, Jhalawar Medical College, Jhalawar, Rajasthan, India.
2 Lecturer, Department of Anatomy, Jhalawar Medical College, Jhalawar, Rajasthan, India.
3 Professor, Department of Anatomy, NIMS, Jaipur, Rajasthan, India.
NAME, ADDRESS, E-MAIL ID OF THE CORESPONDING AUTHOR: Dr. Manoj Kumar Sharma, Associate Professor, Department of Anatomy, III/5, Staff Quarters, Doctors Colony, Medical College Campus, Jhalawar, Rajasthan, Pincode-326001, India.
Phone: 09667334455,
E-mail: drmanojsharma2002@yahoo.co.in
Objectives: In India, especially in Rajasthan, people drink water which contains high level of nitrates and the possibility of finding concentrations of up to 500 mg of nitrate ions per litre of water is not unusual. Excessive use of nitrate fertilisers and herbicides results in accumulation of nitrate in plants and methemoglobinaemia in cattle as consequences of nitrate poisoning. The ingested nitrate is converted to nitrite in the digestive system and it is absorbed in blood, thus causing methemoglobinaemia. Methaemoglobinaemia is not restricted to infants alone, but it is prevalent in higher age groups also.
Methods: Therefore, an experimental study was conducted on 10 rabbits which were between three and a half months to four months of age, which had weights which ranged from 1.310 kg to 1.720 kg. Five groups A, B, C,D and E were formed, with two rabbits in each group. The control Group A was given water orally, which had 45 mg/litres of nitrate. Groups B to E (experimental groups) were administered water orally, which had concentrations of 100mg/litre, 200mg/litre, 400mg/litre and 500mg/litre of nitrate respectively, for 120 days. During experimental period, the differences in general behaviour of rabbits were noted. After this, rabbits were anaesthetised and sacrificed according to guidelines of ICMR and their livers were removed and processed for making paraffin sections,.Hematoxyllin and eosin staining was done for microscopic observations.
Results: During experimental period, the animals were found to be lethargic on 75th day. Quantity of intake of food and water was not altered in the rabbits which were undergoing experiments in different groups. Rabbits of all groups i.e. A to E showed a continuous increase in heart rate (up to 218/minute in Group E) and respiration rate (up to 84/minute in Group E) respectively. The microscopic study showed mild necrosis of hepatocytes, with infiltration of inflammatory cells in between hepatocytes. In higher groups, the liver showed bridging necrosis and portal triditis. Dilatations of central vein with eosinophilic degeneration were observed in Group E only.
Liver, Methaemoglobin, Nitrate, Rabbits, Histopathology, Cyanosis, Nitrite
Introduction
Most of the population of India is exposed to nitrate through ground water and dietary sources [1]. In several developing countries, especially in India, consumption of water which contains high nitrate concentrations, at times, up to 500mg/litre, is not uncommon [2]. Excessive nitrate concentrations in drinking water have been reported to have caused methaemoglobinemia in infants who were upto 6 months of age [3,4]. Maximum permissible limit for nitrate ions in drinking water has been set at 50mg/litre by WHO and at 45 mg/litre by Bureau of Indian Standards (IS-10500) [5–7]. A study which was done on tumourigencity of N-Nitroso compounds, showed incidences of mixed malignant liver carcinoma and endothelial sarcoma when the nitrogenous drugs (200mg/litre) were given for 8 weeks and when sodium nitrite was add to the given drug in olive oil , the animals died shortly after treatment. The microscopic study showed mild degeneration and extensive necrosis of liver cells in centrilobular area [8].
Rabbits were chosen as the experimental animals because their stomach pH was similar to that of infants (3.0–5.0) [9,10]. Oxygen is essential for formation of methemoglobin by nitrite. Nitrate is reduced to nitrite by micro flora in the oral cavity and an increased consumption of nitrite leads to :– increased production of nitrates, excess nitric oxide generation which has vasodilator effects, an enhanced absorption of sodium from intestinal lumen, and increased production of oxygen which will react with other cell constituents, possibly causing irreversible damage [11].
There are three stages of interactions between sodium nitrite and blood: an induction period, a reactionary period and a terminal period , which are often prolonged, during which the product of reactions, chiefly methemoglobin transforms into haematin and other degradation products [12]. These findings indicate that nitrate ingestion creates problems with oxygen carrying capacity of blood [13–16].
So, the health risk which results from exposure to nitrates is therefore related, not only to their concentrations in drinking water and food, but also to conditions which are conducive to their reduction to nitrites [13].
Aim of Study
As per the above reported data, it was planned to study the toxic effects in liver which were caused by exposure of different concentrations of nitrate in drinking water, in an appropriate animal study which was done under laboratory conditions.
Material and Methods
This study was conducted in the Department of Anatomy, S.M.S Medical college and attached group of hospitals, Jaipur, Rajasthan, India on five groups which had 2 rabbits each.
Rabbits were used for the study because their stomach pH was similar to that of infants (pH= 3.0-5.0) [9,10]. The age of rabbits was three and a half to four months and their weights varied from 1.310 kg to 1.720 kg. The groups were identified as A,B,C,D and E. Ad libitum quantity of water which contained 45 mg/litre, 100 mg/litre, 200 mg/litre, 400 mg/litre and 500 mg/litre nitrate (in form of NaNO3) and food which was soaked in the same water were given to groups A to E respectively.
The group which consumed 45mg/litre served as control group. Observations were made during the experimental period of 120 days for the changes in physical activity of the animals, on a pre–designed proforma after every 15 days. After 120 days, the animals were sacrificed according to the guidelines of ICMR17 and they were dissected. Their livers were removed and biopsies were taken from the organs. The tissues were kept in 10% formalin whose volume was 20 times the volume of the tissue. After fixation for 3 days, tissues were passed through an alcohol dehydration series (ascending) and they were then cleared by using xylol. After clearing, the tissues were embedded in melted paraffin for 1 hour and blocks were made. Sections of the blocks were made by using a Rotatory microtome and they were passed through an alcohol dehydration series (descending). They were then stained by Haemotoxylline and Eosin and subjected to a histopathological examination.
Observation and Results
(A) General Observation– Cyanosis appeared in rabbits of Group C, D and E only. The rabbits of Group A did not show lethargy throughout the experimental period. In groups B,C and D, lethargy appeared on 90th day. The rabbits of Group E became lethargic on 60th day. In rabbits of all groups, the heart rate was 140/minute to 144/minute at the start of the experimental period, which increased up to 218/minute in rabbits of Group E after completion of 120 day period [Table/Fig-1]. The respiration rate was noted to be 56/minute to 60/minute in all rabbits on first day of experimental period, which increased up to 84/minute in rabbits of Group E on 120th day of the experiment [Table/Fig-1]. It was observed that tachycardia and tachypnoea preceded the cyanosis, even in groups where lethargy and cyanosis were not present. Increases in heart rate and respiration rate were proportional to the nitrate concentration in the drinking water.
Comparison in Physical activity of Rabbits in all groups during experimental period of 120 Days
| Ive Groups with Animal No. | Heart Rate (135/Min) | Respiration Rate (55/Min) |
|---|
| GPA | GBP | GPC | GPD | GPE | GPA | GBP | GPC | GPD | GPE |
|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
|---|
| Days of observation (120 Days) | 1st Day | 140 | 144 | 140 | 142 | 142 | 140 | 142 | 140 | 142 | 140 | 56 | 58 | 56 | 58 | 56 | 56 | 56 | 58 | 56 | 56 |
| 15th Day | ↑ | – | – | ↑ | – | ↑ | ↑ | – | – | – | ↑ | – | – | – | – | ↑ | – | – | ↑ | – |
| 30th Day | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | – | ↑ | – | ↑ | – | ↑ | ↑ | ↓ | ↑ | ↑ | – |
| 45th Day | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | – | ↑ | – | ↑ | ↑ | ↑ | – | ↑ | ↑ |
| 60th Day | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | – | ↑ | – | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 75th Day | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | – | ↑ | – | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 90th Day | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | – | ↑ | – | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 105th Day | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | – | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| 120th Day | 158 | 158 | 152 | 158 | 166 | 168 | 192 | 198 | 216 | 218 | 60 | 60 | 58 | 60 | 64 | 60 | 64 | 64 | 72 | 84 |
GP = Groups
↑ = Increase in Parameter
↓ = Decrease in Parameter
– = No change was observed
Discussion
The general observations like appearance of cyanosis and lethargy were in accordance with the results of Gupta SK et al., [13], Comly et al., [14], Farrant M et al., [15], and Greenberg et al., [16], which indicated that high nitrate created problems with oxygen carrying capacity of blood.
The findings of present study which were related to heart rate and respiration rate were similar to those of Kielbase et al., [17] and Walton et al., [18]. Kielbase et al., [17] observed that isobutyl nitrate caused a rapid dose dependent and a parallal reduction in systolic and diastolic pressure with an increase in heart rate in experimental animals. Walton et al., [18] observed that in infants, the high nitrate concentration in drinking water produced an increase in respiratory rate.
(B) Histopathological Changes– No changes was observed in livers of rabbits in Group A. Changes appeared in Group B in the form of mild necrosis of hepatocytes and mild infiltration of inflammatory cells in between the hepatocytes [Table/Fig-2]. These changes were more pronounced as the nitrate concentration increased, in the form of bridging fibrosis, which appeared in Group C [Table/Fig-3]. The changes around the portal triad (portal triditis) started in Group B. There was a marked infiltration of inflammatory cells around the portal triad in Group E [Table/Fig-4]. Dilation of central vein and eosinophilic degeneration of sinusoids were observed in Group E only [Table/Fig-5]. Piece meal necrosis and drop out necrosis were also observed [Table/Fig-6]. Kupffer’s cell hyperplasia and bile duct hyperplasia were observed in rabbits of Group E only [Table/Fig-7].
Comparative histopathological changes in liver
| Group A | Group B | Group C | Group D | Group E |
|---|
| Hepatocytes | – | + # | ++ # $ | ++ # # $ | ++++ # # # $$ |
| Portal Triad | – | + | + + | + + | + + + |
| Central Vein | – | – | – | – | – |
| Sinusoid | – | – | – | – | +++ ** |
- = Normal histology
+ = Mild Inflammation
++ = Moderate Inflammation
+++ = Severe Inflammation
# = Mild Necrosis
## = Moderate Necrosis
### = Severe Necrosis
$ = Bridging fibrosis
** = Neutrophilic Infiltration
Microphotograph of liver showing periportal triditis, piece meal necrosis and drop out necrosis (10 X)
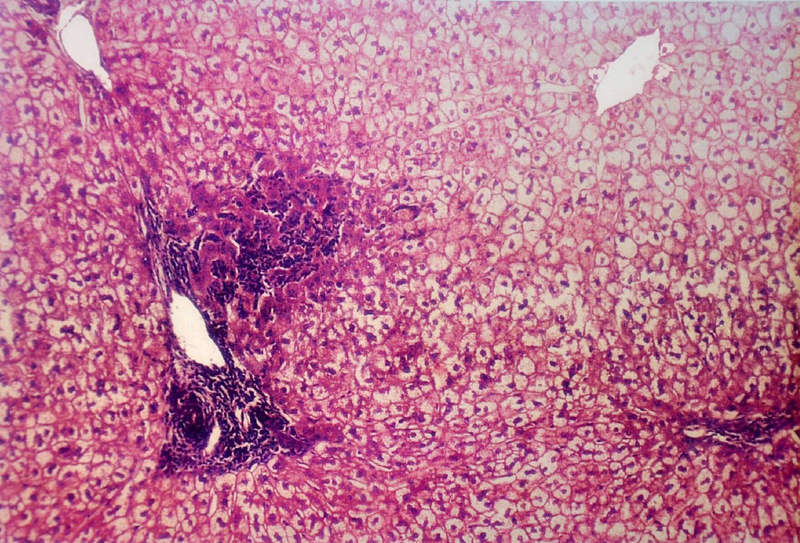
Microphotograph of liver showing piece meal necrosis with periportal triditis (10 X)
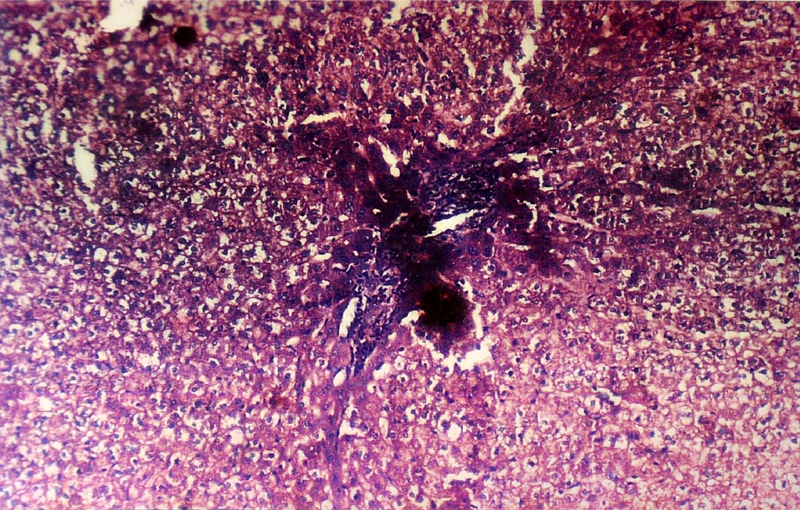
Microphotograph of liver showing piece meal necrosis and bridging fibrosis (10 X)
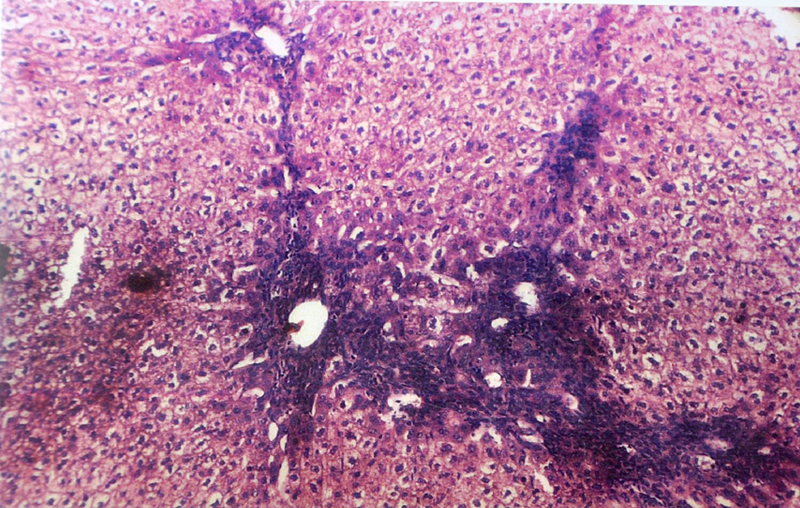
Microphotograph of liver showing piece meal necrosis and periportal triditis in high power (40 X)
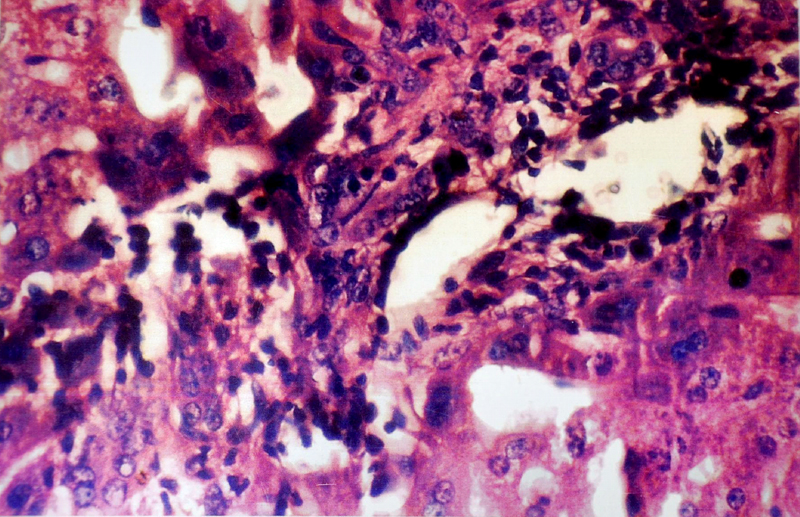
Microphotograph of liver showing Kupffer’s cell hyperplasia in high power (40 X)
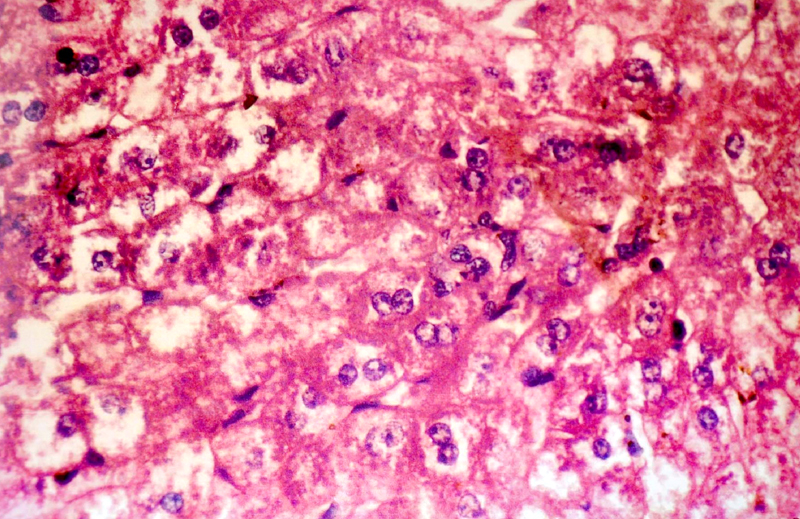
The findings of present study were in accordance with the observations of Hille I Shuval et al., [19], Litvinov NN et al., [20], Gataseva P et al., [21], Gilman AP et al., [22], Farrant et al., [15] and Ogur R et al., [22], who observed that a high nitrate administration caused hepatocellular degeneration with increased intercellular spaces between liver cells in Groups B and C.
These findings showed clearly that high nitrate ingestion could cause pathological changes in liver histology.
Conclusion
The results of present study proved a strong interdependence between high nitrate concentrations and changes in physical activities, with histopathological changes in livers of rabbits. The degree of damage was more pronounced as nitrate concentration increased in drinking water. The possible cause could be the reversal of cyt.b5 reductase activity and its adaptation with increasing water nitrate concentrations, for compensating methaemoglobinaemia. While an isolated study cannot be extrapolated to humans, it highlights the need for conducting further studies on populations which consume nitrate rich water.
The nitrate problem has not been taken up seriously in our country. It is expected that the findings of this study will draw attention of decision makers to take note of this serious problem and to take adequate steps to ensure that safe drinking water is available to public.
Pathophysiology of Nitrate Toxity
The essential action in the formation of methemoglobin is oxidation of the ferrous ion to ferric ion. This action may be brought about in one of the following ways–By direct action of the oxidant or by the action of a hydrogen donor in the presence of oxygen or by auto oxidation. In the presence of nitrites, the ferrous ion of haemoglobin gets directly oxidised to ferric state. Normally, the methemoglobin which is is formed is reduced by the following reaction:
Hb+3 +Red.Cyt b5 ----------> Hb+2 + Cyt b5
Reduced cytohrome b5 is generated by the enzyme cyt.b5 reductase:
Oxy cytb5 + NADH ----------> Red cyt b5 + NAD.
Thus, the enzyme, cyt b5 reductase plays a vital role in counteracting the effect of nitrate ingestion [23].
Bacteria which cause non–specific diarrhoea are generally considered to be responsible for conversion of nitrate to nitrite. Lower stomach pH of adults acts as an inhibitor of these bacteria. However, they can multiply in relatively high pH of the stomach.
GP = Groups↑ = Increase in Parameter↓ = Decrease in Parameter– = No change was observed
- = Normal histology+ = Mild Inflammation++ = Moderate Inflammation+++ = Severe Inflammation# = Mild Necrosis## = Moderate Necrosis### = Severe Necrosis$ = Bridging fibrosis** = Neutrophilic Infiltration
[1]. Cornblath M, Hartmann AF, Methemoglobinemia in young infantsJ Pediat 1948 33:421-25. [Google Scholar]
[2]. Jaffe ER, MethemoglobinemiaClinics in Hematology 1981 10(1):99 [Google Scholar]
[3]. Marshall CR, Marshall W, Action of nitrates on bloodJ Biol Chem 1945 158:187-208. [Google Scholar]
[4]. Knotek Zand Schmidt P, Pathogenesis, incidence and possibilities of preventing alimentary nitrate methemoglobinemia in infantsPediatrics 1964 34:78-83. [Google Scholar]
[5]. WHO. Guidelines for dinking water quality, Volume 1.Genava.1993;52-53 [Google Scholar]
[6]. Drinking water specification. IS 10500:1991, Bureau of Indian standards, New Dehli.1995;3? [Google Scholar]
[7]. Li H, Duncan C, Townend J, Kilham K, Smith JM, Johnston P, Nitrate reducing bacteria on rat tongue. Applied and environmentalMicrobiology 1997 63:924-30. [Google Scholar]
[8]. Goodall CM, Lijinsky W, Tomatis L, Tumorigenicity of N-NitrosohexamethyleneimineCancer Research 1968 28(7):1217-20. [Google Scholar]
[9]. World Health Organization. Nitrate, nitrite and N-Nitroso compounds. Environmental Health Criteria 5, Geneva.1977;15 [Google Scholar]
[10]. WHO. Nitrates, nitrites and N-Nitroso compounds. Environmental Health criteria 5, Genava.1977;77 [Google Scholar]
[11]. Epidemiological Evaluation o Nitrate toxicity and DPNH dependent Methemoglobin Diaphorase activity in infants. SMS Medical College and NEERI Zonal Laboratory, Jaipur. Project report, council of Scientific and Industrial Research, New Dehli.1994-19970 [Google Scholar]
[12]. Gupta SK, Gupta RC, Epidemiological evaluation of recurrent stomatitis, nitrates in Drinking water and cytochrome b5 reductase activityThe American Journal of Gastroentrology 1999 94(7):1808-12. [Google Scholar]
[13]. World Health OrganizationGuidelines for research on acute respiratory infection. Memorandum from a WHO MeetingBulletin of the World Health Organization 1982 60:521-33. [Google Scholar]
[14]. Comly HH, Cyanosis in infants caused by nitrates in well waterJAMA 1945 129:112-16. [Google Scholar]
[15]. Ferrant M, Methemoglobinemia. Two cases in new born infants caused by nitrates in well waterThe Journal of Pediatrics 1945 :585-91. [Google Scholar]
[16]. Greenberg M, Birnkrant WB, Schiftner JJ, Outbreak of sodium nitrite poisoningAmerican Journal of Public Health 1945 (35):1217-20. [Google Scholar]
[17]. Kielbase W, Fung HL, Nitrite inhalation in rats elevates tissue NOS III expression and alters tyrosin nitration and phosphorylationBiochemical and Biophysical Research Communications 2000 275(2):335-42. [Google Scholar]
[18]. Walton G, Survey of literature relating to infants methemoglobinemia due to nitrate contaminated waterA.J.P.H 1951 (41):986-96. [Google Scholar]
[19]. Shuval HI, Gruener N, Epidemiological and toxicological aspects of nitrates and nitrites in the environmentAm J Public Health 1972 62:1045-52. [Google Scholar]
[20]. Litvinov NM, Lamentova TG, Kazachkov VI, Structural and functional changes in the liver of pregnant rats and their fetuses exposed to cadmium, benzol and lead nitrateGigiena I Sanitariia 1991 (5):19-23. [Google Scholar]
[21]. Getseva P, Lazarova A, Maximova S, Pavlova K, Experimental data on the effect of nitrates entering the organism with the drinking waterFolia Media 1996 38(1):75-83. [Google Scholar]
[22]. Ogur R, Coskun O, Korkmaz A, Oter S, Yaren H, Hasde M, High nitrate intake impairs liver functions and morphology in rats; protective effects of α-tocopherolEnviron Toxicol Pharmacol 2005 Jul 20(1):161-6.doi: 10.1016/j.etap.2004.12.051 [Google Scholar]
[23]. World Health Organization. Respiratory infection in children. Management in small hospitals. Manual for Doctors. WHO. Geneva. 1998 [Google Scholar]