It is estimated that 120 million people worldwide are affected by filariasis. In the South East and South Asian region, Wuchereria bancrofti is the most prevalent parasite causing filariais in 99.4 % of cases. In the present case the parasite was a cause of unexplained ascitis in a young female whose previous cytological report was suspicious in nature. Extensive physical and radiological assesment was not able to determine the cause of ascitis. Repeat cytological examinantion revealed a few microfilariae of Wuchereria bancrofti. The patient was diagnosed as a case of low density microfilaremia and was put on anti-filarial therapy subsequent to which ascitis of the patient regressed. In this case, the clinical picture did not give a clue regarding the aetiology of the ascites. There was no evidence suggestive of filariasis, such as lymphoedema or hydrocele except mild eosinophilia. Circumstantialtial evidence regarding the aetiology of the effusion in our patient included her recovery following antifilarial therapy. The cytological diagnosis of Mf in this patient avoided further expensive investigations and the patient responded to antifilarial therapy.
Low density microfilaraemia, Ascitis
Introduction
It is known that microfilaria (Mf) affects an estimated 1.5 million people worldwide. The south – east Asian region contributes to about 67% of the global burden. India alone contains 74% of the endemic population. Of all the species which cause filariasis, Wuchereria bancrofti is the most prevalent one (99.4% cases) [1]. Mf has been localised in aspirates from unusual sites like – thyroid nodules, gingival and arm swellings [2,3] breast aspirates, nipple secretions, cervico – vaginal smears, ascitic fluid, pleural fluid, pericardial fluid and even cerebrospinal fluid [4]. Usually, such a presence is associated with a minimal reactive change or loss of function. In the present case, a 36 year old female presented to the medicine outpatients department of a tertiary care centre, complaining of an abdominal distension of unknown aetiology (with a previous cytological report which had a suspicious nature) and low grade fever. Various diagnostic tests were conducted to determine the cause of the ascitis and a chance finding of microfilaria in cytological smears which were made from repeat paracentesis, led to the final diagnosis and alleviation of the symptoms in the patient.
Case Report
A 36 year old female presented to the medicine outpatients department of a tertiary care centre with complaint of abdominal distension of 3 months duration. A previous cytological examination of the ascitic fluid which was done a month ago was of suspicious nature, with atypical cells being reported in the aspirate. Physical and radiological examination confirmed ascites. A diagnostic paracentesis was done at the time of admission and the cytological smears concurred with the previous report of atypical/suspicious cells. A detailed radiological assessment was done (sonography, radiograms, mammogram and computed tomography), which failed to reveal any suspicious mass or lesion. A battery of serological, biochemical and haematological investigations which were done (including VDRL test – Venereal Disease Research Laboratory test and viral markers for hepatitis B and C and HIV) were either within normal limits or unremarkable. A routine haemogram which was done, showed a leukocyte count of 10800 and a differential leukocyte count of 70% polymorphs, 16% lymphocytes, 02% monocytes and 12% eosinophils, without any evidence of haemoparasites, which included malarial parasites and immature cells. The aetiology, thus remained unexplained.
A repeat paracentesis was done after much discussion, after a week, to ascertain the nature of the aspirate. The fluid was centrifuged and this time, multiple smears were made from the deposits and whole of the fluid was used up. Some of them were air dried and stained with May Grunwald’s Giemsa (MGG) stain and the rest were wet fixed and stained with haematoxylin and eosin. The centrifuged smears demonstrated small groups and clusters of reactive mesothelial cells, lymphocytes, a few polymorphs and a fair number of eosinophils. A few isolated mesothelial cells also showed a high nuclear:cytoplasmic ratio, with an irregular nuclear contour, hyperchromasia and prominent nucleoli. Cytoplasm in these cells was minimal and basophilic in appearance [Table/Fig-1]. An incidental finding in the form of a single microfilaria, was identified by the presence of its sheath, its smooth graceful curves, elongated terminal nuclei and pointed posterior ends [Table/Fig-2]. Subsequent examination of all the centrifuged smears also revealed one to two microfilaraemia, but only in few of the smears. The patient was further investigated and three consecutive night – time blood samples which were obtained by finger prick, failed to demonstrate microfilaria. However; microfilaria was detected when the patient’s 1 ml venous blood was processed by membrane filtration technique. Hence, it was therefore concluded that the patient was suffering from low density microfilaraemia which presented with ascitis, with no clinical symptom of filariasis.
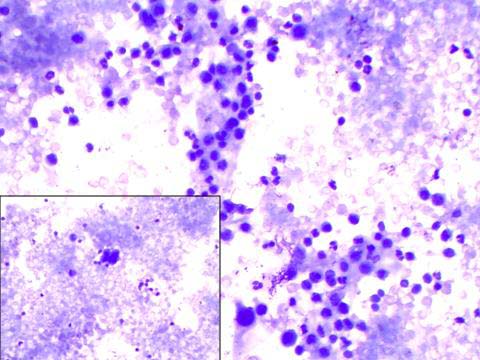
Cytological smear showing reactive atypia in mesothelial cells with lymphocytes, eosinophils and neutrophils (MGG X 200). Inset Atypical looking mesothelial cells (MGG X 400)
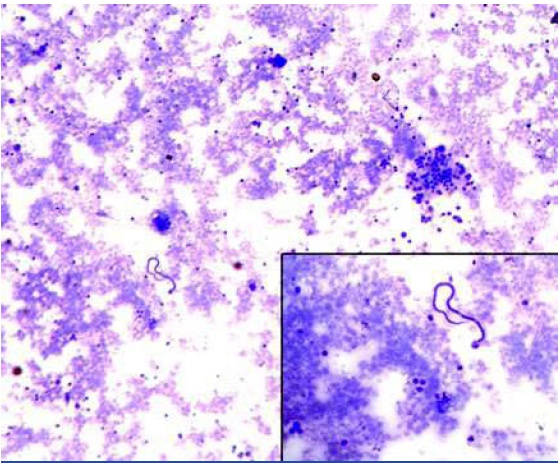
Cytological smear showing a single microfilaria of Wuchereria bancrofti along with a cluster of reactive looking mesothelial cells (MGG X 100). Inset microfilaria at a higher magnification (MGG X 400)
It was decided at a clinico-pathological meet, to treat the patient with diethylcarbazine. After 4 weeks, the lady was found to be asymptomatic, as the amount of ascitic fluid regressed gradually, along with an improvement in her general profile. She continued to be asymptomatic on her subsequent follow-up visits (1 year), after the initial diagnosis. In this case, the clinical picture did not give a clue regarding the aetiology of the ascites. There was no evidence which was suggestive of filariasis. Circumstantial evidence regarding the aetiology of the effusion in our patient included her recovery following the antifilarial therapy. The cytological diagnosis of Mf in this patient prevented further expensive investigations and the patient responded to the antifilarial therapy.
Discussion
It has been estimated that filariasis, as a cause of mortality and morbidity, affects more than 40 million individuals [5]. Studies from the Indian subcontinent have shown that it is also a great economic burden to the susceptible population, costing the Indian treasury upto 842 million per year [6].
Filarisis has been reported in various body fluids and sites. The clinical presentation of lymphoedema, elephantiasis or eosinophilia usually accompanies in most of the cases.
In the present case, the clinical picture did not reveal any association between ascitis and filariasis, except for eosinophilia (with no skin changes, limb oedema or wheezing/crackling of the chest). This was more so confounded by the unremarkable haematological and radiological examinations, along with the suspicious cytological smear report.
It was presumed that in the present case, the microfilaria caused ascitis through disruption of the lymphatic system, which may occur because of repeated injuries or obstruction (fibrosis), which are caused by repeated inflammatory episodes, as has been described in the literature [7].
The patient was also categorised as suffering from “low density microfilaraemia”- a term which is usually applied to those densities which are detected by the nucleopore method, but not by the more usual finger-prick method, as was seen in the present case report. This could also be an explanation for the low parasitic yield in the ascitic fluid [8].
Apart from the 3 consecutive midnight samples, sampling after a DEC stimulation and micropore/membrane filter concentration techniques; some newer ancillary investigations have been made available to detect filarial antigen. The presence of circulating filarial antigen in the peripheral blood, with or without microfilariae, is now considered diagnostic of a patent filarial infection and it is also used to monitor the effectiveness of therapy. Commercial kits are available to test the venous blood and they can be quantitative (enzyme-linked immunoassay [ELISA]) Og4C3 monoclonal antibody–based assay) or qualitative (immunochromatographic). The former is one of the best predictors of worm burden [9]; the latter, although not as sensitive [10], was once considered the test of choice in field surveys. However, results from this test remain positive for 3 years post treatment; hence, this test has been shown to be ineffective for the same reason [11].
The usual IgG and IgE lack specificity (species differentiation) and they usually cross react with antigens of Strongyloides. In addition, they do not differentiate between past and recent infections. Thus, a diagnosis which is based on recombinant antigens is useful only in expatriates but not in those who live in endemic regions. All these ancillary investigations were not employed in the present study, due to demonstrable microfilariae, first in the repeat paracentesis and then in micropore concentration technique.
Apart from haematological and the newer ancillary immunological tests which have been described above, a radiological assessment is also added in the diagnostic protocol, as it sometimes yields some important information. Chest skiagrams often reveal diffuse pulmonary infiltrates (especially in patients with tropical eosinophilia). Ultrasonography is useful in cases with lymphatic obstructions in the inguinal and scrotal lymphatics, which can be demonstrated and monitored with ultrasonography. Ultrasonography has also been used to demonstrate viable worms, which are seen to be in continuous motion (i.e., “filarial dance” sign). This imaging characteristic has been used to monitor the effectiveness of the treatment [12].
The cytological diagnosis of Mf in this patient prevented further expensive investigations and the patient responded to antifilarial therapy.
[1]. http://www.searo.who.int/entity/vector_borne_tropical_diseases/topics/lymphatic_filariasis/en/index.html [accessed 19 June 2013] [Google Scholar]
[2]. Azad K, Arora R, Gupta K, Sharma U, Lymphatic filariasis: Aspiration of adult gravid female worm from a soft tissue swellingJ Cytol 2010 27:156-57. [Google Scholar]
[3]. Pahwa R, Arora VM, Microfilaria in cytology smears from upper arm swellingJ Cytol 2010 27:155-56. [Google Scholar]
[4]. Tewarson SL, Mehrotra R, Singh M, Mannan R, Detection of microfilaria of Wuchereria bancrofti in cerebro spinal fluidCytopathol 2007 18:393-94. [Google Scholar]
[5]. Babu BV, Swain BK, Rath K, Impact of chronic lymphatic filariasis on quantity and quality of productive work among weavers in an endemic village from IndiaTrop Med Int Health May 2006 11(5):712-17. [Google Scholar]
[6]. Ramaiah KD, Das PK, Michael E, Guyatt H, The economic burden of lymphatic filariasis in IndiaParasitol Today Jun 2000 16(6):251-53. [Google Scholar]
[7]. Andres Cardenas, Chopra S, Chylous AscitesAmerican J of Gastro 2002 97(8):1896-900. [Google Scholar]
[8]. Kimura E, Penela L, Samarawickrema , Spears GFS, Low density microfilaremia in SamoaBulletin of the World health Organization 1985 63(6):1089-96. [Google Scholar]
[9]. Chanteau S, Moulia-Pelat JP, Glaziou P, Nguyen NL, Luquiaud P, Plichart C, Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasisJ Infect Dis Jul 1994 170(1):247-50. [Google Scholar]
[10]. Nguyen NL, Plichart C, Esterre P, Assessment of immunochromatographic test for rapid lymphatic filariasis diagnosisParasite Dec 1999 6(4):355-58. [Google Scholar]
[11]. Schuetz A, Addiss DG, Eberhard ML, Lammie PJ, Evaluation of the whole blood filariasis ICT test for short-term monitoring after antifilarial treatmentAm J Trop Med Hyg Apr 2000 62(4):502-03. [Google Scholar]
[12]. Dreyer G, Noroes J, Amaral F, Nen A, Medeiros Z, Coutinho A, Direct assessment of the adulticidal efficacy of a single dose of ivermectin in bancroftian filariasisTrans R Soc Trop Med Hyg Jul-Aug 1995 89(4):441-43. [Google Scholar]