The term “Small Round – Cell Tumours” (SRCT) applies to a group of highly aggressive malignant neoplasms which feature the predominantly small and monotonous un – differentiated cells with high nucleocytoplasmic ratios on histology. This group includes Ewing’s Sarcoma (ES), Primitive Neuroectodermal Tumour (PNET) or extraskeletal Ewing’s sarcoma, neuroblastoma, rhabdomyosarcoma, desmoplastic small round cell tumour, non – Hodgkin’s lymphoma, small - cell osteosarcoma, small – cell carcinoma (either undifferentiated or neuroendocrine), olfactory neuroblastoma and mesenchymal chondrosarcoma. Their clinical presentations often overlap, thus making the diagnosis problematic in some cases.
Considering the fact that both the treatment and the prognosis vary greatly among these tumours, a conclusive diagnosis is essential. The anatomical locations and the microscopic details of these tumours and other aspects of their clinical presentations have a strong bearing on the relative likelihood of their respective diagnoses, and their immunohistochemical analyses may be tailored according to such considerations [Table/Fig-1].
Several monoclonal antibodies have been developed, that detect different epitopes of the antigens which are present in the tumour cells. However, there is no antibody which is specific for a single tumour type and the pathologist has to judiciously correlate the clinical, radiological and the morphological findings with a panel of immumohistochemical (IHC) markers.
Material and Methods
Formalin – fixed, paraffin-embedded sections of all the cases which were diagnosed as small round – cell tumours on small biopsies and resected specimens were retrieved from the files of the Department of Pathology of Sri Ramachandra Medical College and Research Institute, in the period from January 2005 to December 2009. The patients belonged to all the age groups were included in the study. Decalcification was performed on the bony tissues before the routine processing was done.
Haematoxylin and eosin stained sections of all the study cases were retrieved to confirm the tissue diagnosis and also the immunohistochemical stained sections were studied to categorise the tumours.
Inclusion Criteria: Small round cell tumours of the soft tissues and the bone were only included in the study.
Exclusion Criteria: Small round cell tumours of the bone marrow, spleen and the lymph node were excluded from the study.
The immunophenotypic panel of markers which were used in the study to differentiate and categorise the small round blue cell tumours were-CD45/LCA (the lymphocyte common antigen), CD20, CD3, CK, CD99, desmin, EMA (epithelial membrane antigen), synaptophysin, chromogranin, GFAP (Glial fibrillary acidic protein).
Results
This study was designed to evaluate and analyse the small round cell tumours, based on their morphologies and immunohistochemical profiles. A total of 43 cases of small round cell tumours which were identified and studied, have been presented in [Table/Fig-2].
Frequency and Percenctage of Cases
| Diagnosis | No of cases | Percent |
|---|
| Atypical carcinoid | 3 | 7.0 |
| EWINGS / PNET | 6 | 14.0 |
| Hepatoblastoma | 1 | 2.3 |
| Medulloblastoma | 1 | 2.3 |
| Neuroblastoma | 2 | 4.7 |
| NHL | 19 | 44.2 |
| Olfactory neuroblastoma | 3 | 7.0 |
| Rhabdomyosarcoma | 2 | 4.7 |
| Small (oat) cell carcinoma | 1 | 2.3 |
| Small cell osteosarcoma | 1 | 2.3 |
| Synovial sarcoma | 2 | 4.7 |
| Wilms tumor | 2 | 4.7 |
| Total | 43 | 100.0 |
In the present study, the ages of the patients with small round cell tumours ranged from 5 days to 73 years. Most of the tumours (44%) had occurred in the age group of 15-45 years and 21% of the tumours had occurred in the age group of above 60 years The age wise distribution of the cases as males and females has been presented in [Table/Fig-3].
Age-wise and Sex-wise distribution of cases
| Age group (years) | Total |
|---|
| < 5 | 5 to 15 | >15 to 45 | >45 to 60 | > 60 |
|---|
| Male | 7 | 3 | 12 | 3 | 7 | 32 |
| % within Sex | 21.9% | 9.4% | 37.5% | 9.4% | 21.9% | 100.0% |
| % within Age group | 87.5% | 100.0% | 63.2% | 75.0% | 77.8% | 74.4% |
| Female | 1 | 0 | 7 | 1 | 2 | 11 |
| % within Sex | 9.1% | 0% | 63.6% | 9.1% | 18.2% | 100.0% |
| % within Age group | 12.5% | 0% | 36.8% | 25.0% | 22.2% | 25.6% |
| Total | 8 | 3 | 19 | 4 | 9 | 43 |
| % within Sex | 18.6% | 7.0% | 44.2% | 9.3% | 20.9% | 100.0% |
| % within Age group | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% | 100.0% |
Among the 43 cases, in 40 cases, a panel of monoclonal antibodies was utilised, and we were able to arrive at a final diagnosis for all these cases. Among the remaining three cases, 2 cases were Wilm’s tumour and 1 case was a hepatoblastoma. These 3 cases were diagnosed on haematoxylin and eosin stained sections on the basis of the morphology alone and immunohistochemistry was not done for these cases.
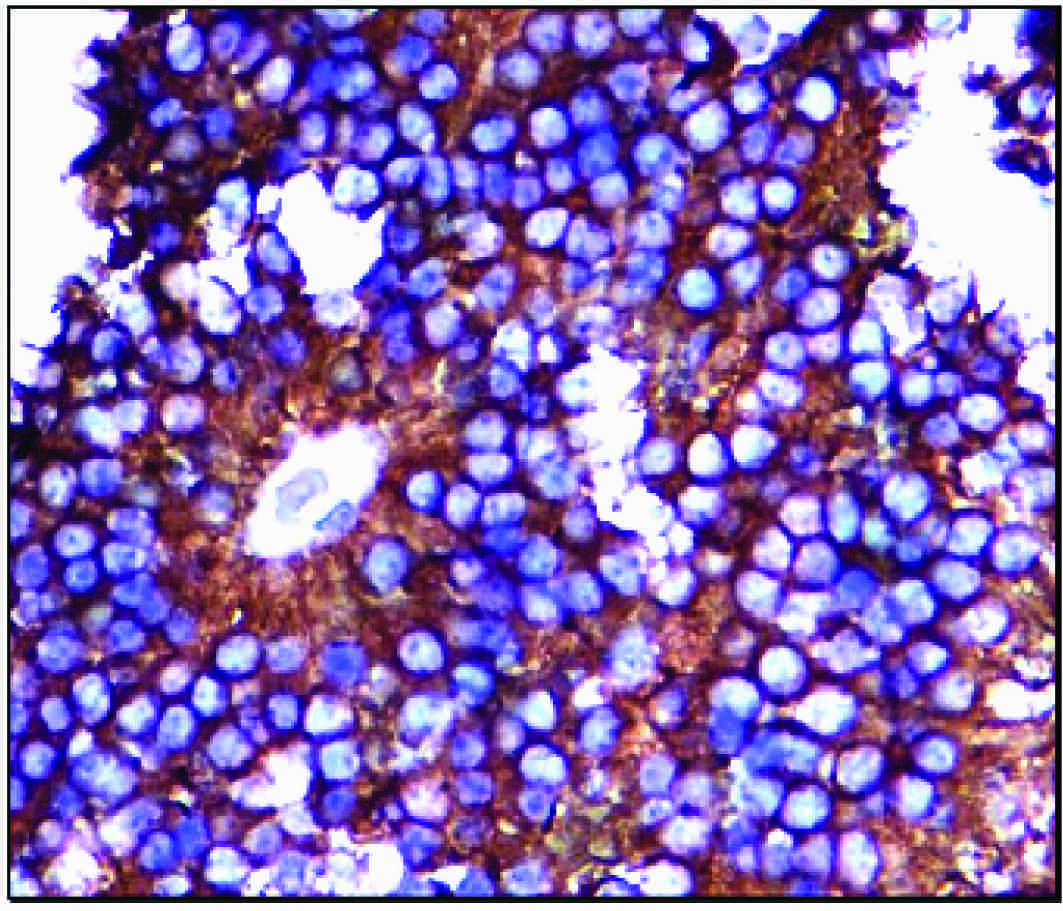
Among the 40 cases which were stained by immunohistochemical methods, 19 were positive for CD45, which were further categorised into the T or B cell types by using CD 19 and CD3 and they were diagnosed as the Non-Hodgkin’s lymphoma B (12) and T (7) cell types respectively [Table/Fig-4a and b]. One case of Non-Hodgkin’s lymphoma showed focal positivity for CD99. Six cases were positive for CD99 and they were diagnosed as Ewing’s/PNET [Table/Figs-5a and b]. A single case of small cell osteosarcoma showed positivity for S100 and a focal positivity for CD99. One of the two cases of monomorphic synovial sarcomas also expressed the CD99 positivity. Synaptophysin and chromogranin positivities were noted in all the cases of atypical carcinoids and in three cases of olfactory neuroblastoma (esthesioneuroblastoma) [Table/Figs-6a and b], which has been presented in [Table/Fig-7].
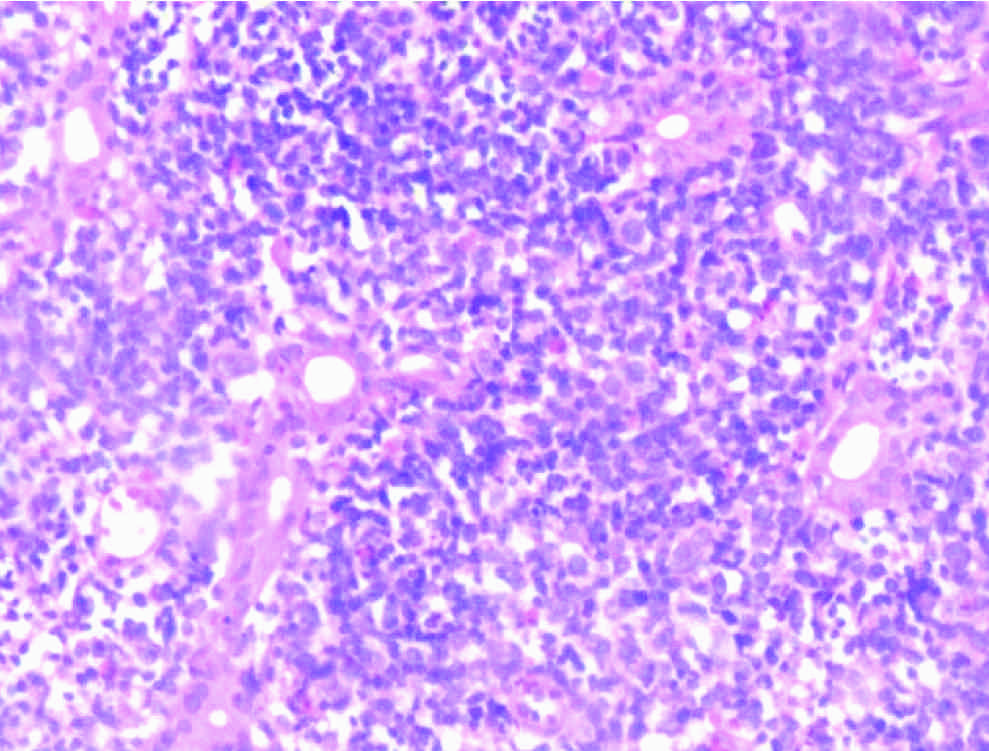
Non-Hodgkins lymphoma (duodenum). Monomorphic small to medium cells.
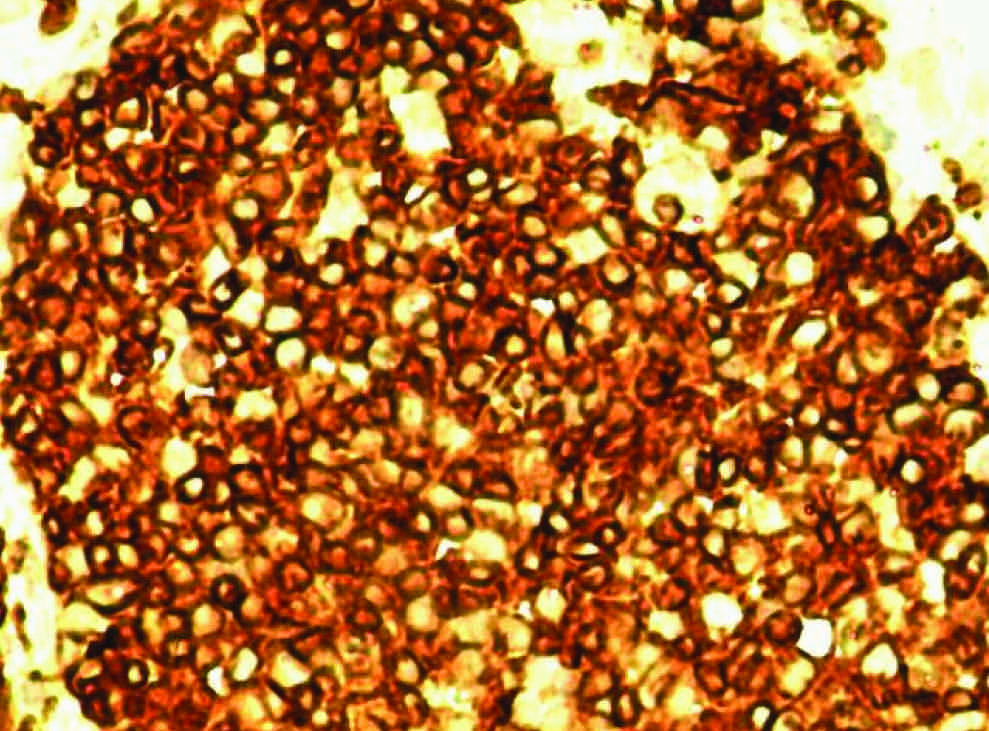
CD3 Immunoexpression in a T-cell lymphoma of the small intestine
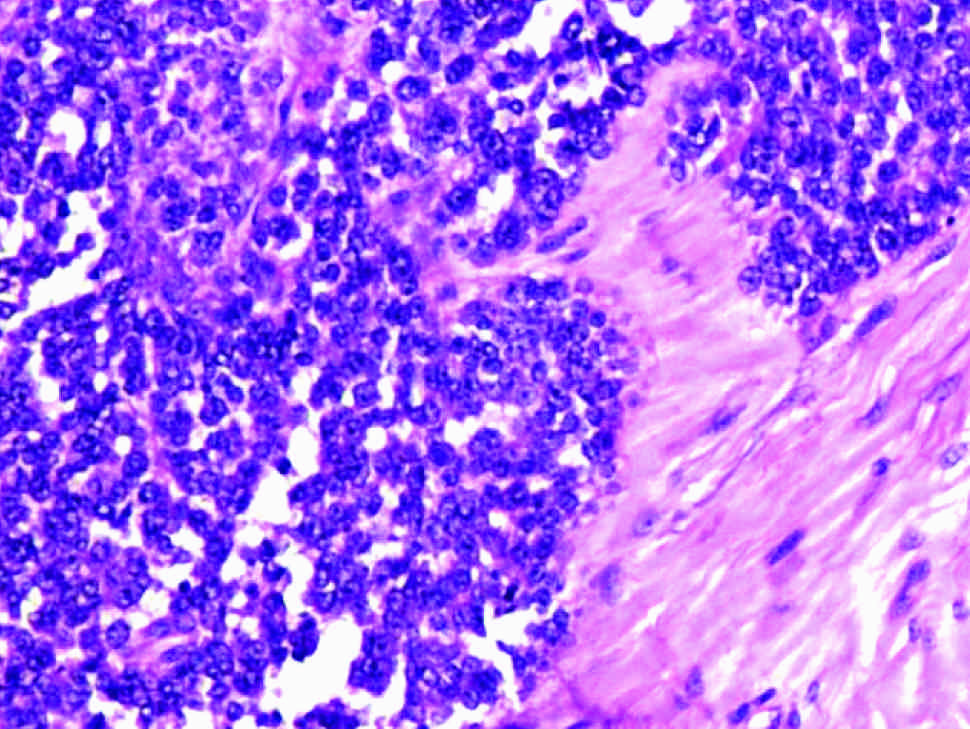
Ewing sarcoma phalynx. Cells with uniform round nuclei
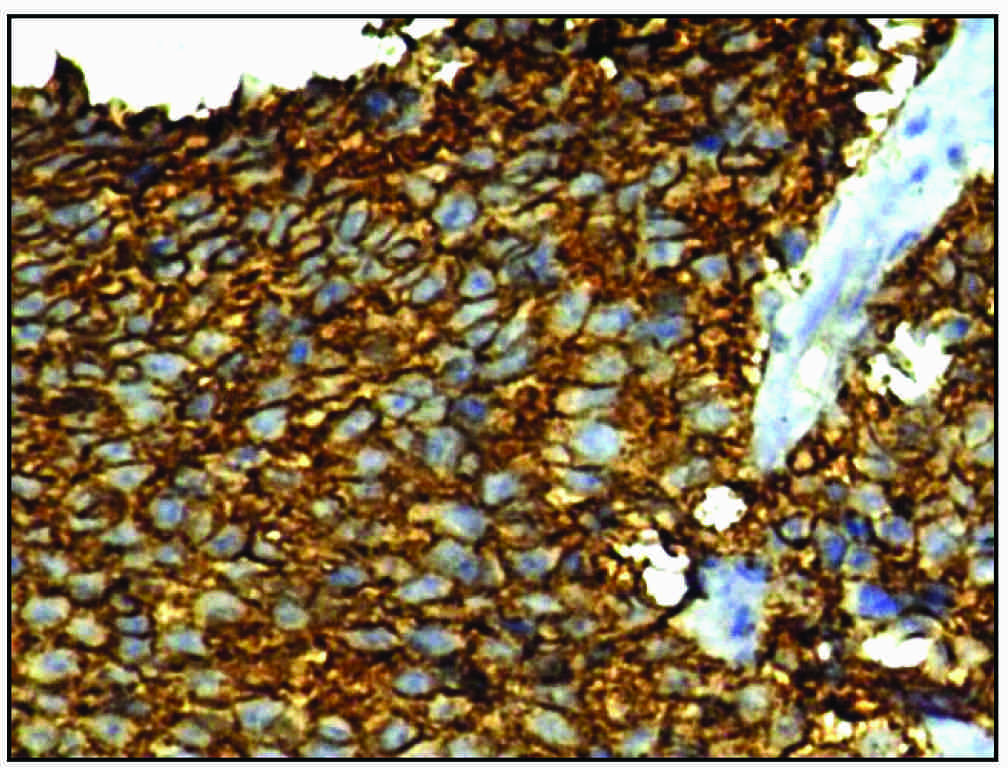
Immunohistochemical expression of CD99 showing characteristic reactivity on the cell membranes
Olfactory neuroblastoma. The “small blue round cell neoplasm” with scant cytoplasm surrounding variably hyperchromatic nuclei and occasional rosette formation
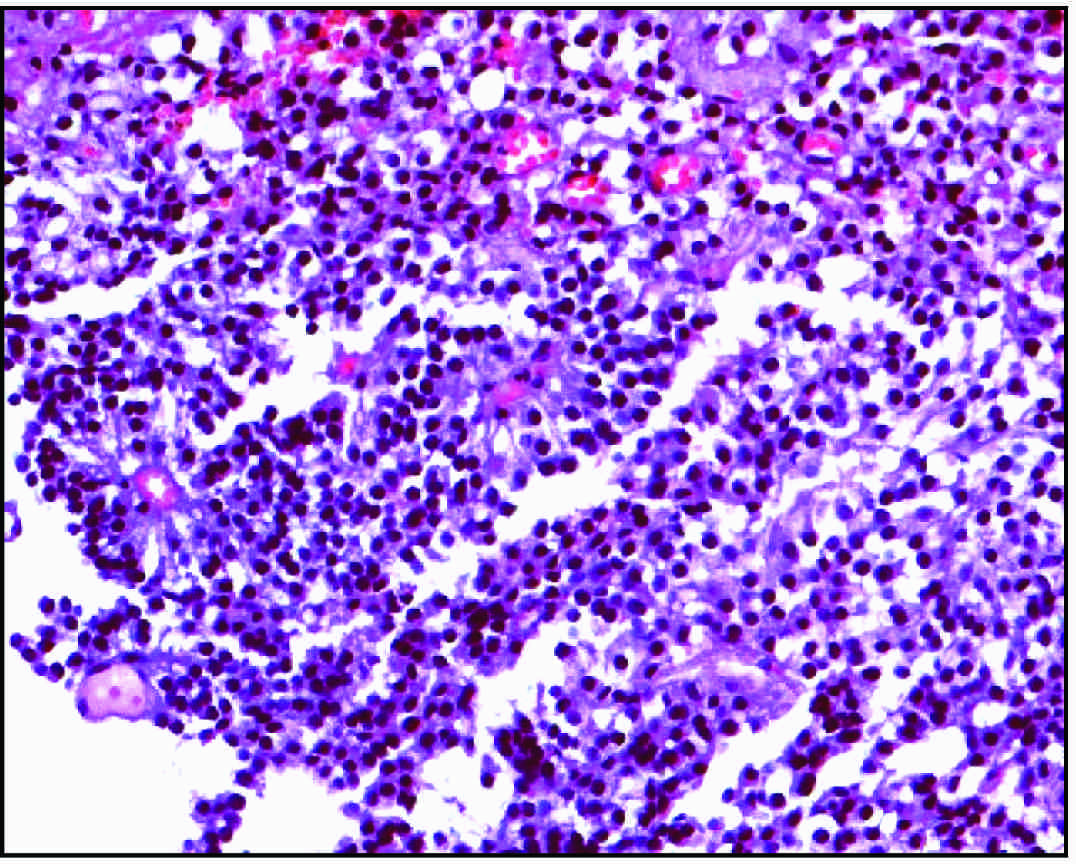
Immunohistochemical expression of synaptophysin showing characteristic reactivity on the cell membranes
Immunohistochemical profile of a spectrum of small round cell tumor LL-lymphoblastic lymphoma, DLBCL-diffuse large B-cell lymphoma, NHL-non-Hodgkin’s lymphoma, RMS rhabdomyosarcoma, ONB-olfactory neuroblastoma, PNET-primitive neuroectodermal tumor; + moderately positive, ++ strongly positive
| Age (years/Months/days) | Sex | Tissue | Initial Diagnosis | Panel of Monoclonal Antibodies | FINAL DIAGNOSIS |
|---|
| CD45 | CD3 | CD20 | CD99 | CK | VIMENTIN | DESMIN | EMA | SYNAP | CHROMO | S100 | GFAP |
|---|
| 60 | M | testicular mass (rt) | NHL, poorly differentiated ca | + | - | + | | - | | | | | | | | NHL, B cell type |
| 37 | M | duodenal Bx | NHL, poorly differentiated ca | + | ++ | - | | - | | | | | | | | NHL, T-cell type |
| 65 | M | stomach growth | NHL, poorly differentiated ca | ++ | - | ++ | | - | | | | | | | | NHL, B-cell type |
| 21 | M | Bone & soft tissue growth | NHL, Ewings/PNET | ++ | - | + | - | | | | | | | | | NHL, B cell type |
| 19 | M | mediastinal mass | NHL, poorly differentiated ca | + | + | - | | - | | | | | | | | NHL, T cell type |
| 3 years & 4 months | M | maxilary sinus growth | NHL, poorly differentiated ca, NHL | + | + | - | | - | | | | | | | | NHL, T cell type |
| 12 | M | mediastinal mass | NHL | + | + | - | | | | | | | | | | LL, T cell |
| 14 | M | Anteriomediastinal | NHL, poorly differentiated ca | ++ | + | | | - | | | | | | | | LL, T cell |
| 19 | F | thigh mass | NHL,poorly differentiated ca, Ewings/PNET | + | Focal + | + | | | | | | | | | | NHL, B-cell type |
| 63 | M | caecum | NHL | + | | + | | | | | | | | | | DLBCL |
| 29 | M | ileal mass | NHL, poorly differentiated ca | + | - | + | | - | | | | | | | | NHL, B cell type |
| 60 | F | parotid gland | NHL, poorly differentiated ca | + | - | + | | - | | | | | | | | NHL, B cell type |
| 62 | F | retroperitonel mass | NHL | + | scattered+ | + | | | | | | | | | | NHL, B cell type |
| 35 | M | left frontal mass | NHL, Metastatic ca | ++ | - | ++ | | - | | | | - | - | - | - | NHL, B-cell type |
| 6 | M | pelvic mass | NHL, PNET, RMS | ++ | scattered+ | ++ | - | | | - | | | - | | | NHL, B-cell type |
| 5 | M | C 6-7 epidural mass | NHL, PNET, RMS | + | | + | Focal + | | | - | | | | | - | NHL, B cell type |
| 38 | M | skin nodules | NHL | + | + | - | | | | | | | | | | NHL, T cell type |
| 72 | M | maxillary growth | NHL, poorly differentiated ca | + | ++ | - | | - | | | | | | | | NHL, T- cell type |
| 70 | M | left frontotemporal | NHL | + | scattered+ | + | | | | | | | | | - | NHL, B cell type |
| 62 | M | soft & bony tissue | Synovial sarcoma, Ewings/PNET | | | | + | | | | + | | | Focal + | | Synovial sarcoma (monomorphic) |
| 64 | M | soft tissue thigh | Soft tissue sarcoma, RMS | | | | | | + | - | + | | | - | | Synovial sarcoma (monomorphic) |
| 16 | F | mediastinal tumor biopsy with secondaries in heart | Atypical carcinoid | | | | | | | | | ++ | ++ | | | Atypical carcinoid |
| 62 | M | short resection of duodenum | Atypical carcinoid | | | | | + | | | | + | + | | | Atypical carcinoid |
| 52 | M | gastric biopsy | Atypical carcinoid | | | | | | | | | + | + | | | Atypical carcinoid |
| 25 | M | renal biopsy | Ewings/PNET, NHL | - | | | ++ | - | | | | | - | | | EWINGS / PNET |
| 43 | F | left lung lower lobe | Ewings/PNET, NHL, poorly differentiated ca | - | - | - | ++ | - | | | | | | | | PNET |
| 3 | M | mass oral cavity | Ewings/PNET, NHL, RMS, Poorly differentiated ca | - | - | - | + | - | | - | | | | | | PNET |
| 16 | F | excised tissue from clavicle | Ewings/PNET, NHL | - | | | + | | | | | | | | | EWINGS/PNET |
| 28 | M | soft tissue thigh | Ewings/PNET, NHL, RMS | | | | ++ | | + | - | - | | - | - | | EWINGS / PNET |
| 19 | M | bone specimen right little finger | Ewings/PNET, NHL | - | | | ++ | | | | | | | | | EWINGS / PNET |
| 41 | M | mass with attached rib | Small cell Osteosarcoma, Ewing/PNET | | | | Focal + | | | | | | | ++ | | Small cell osteosarcoma |
| 73 | F | Sphenoidal mass | ONB, NHL | - | | | | | | | - | ++ | ++ | - | - | ONB Gr-I |
| 47 | M | maxillary antrum | ONB, NHL | - | | | | | | | - | ++ | | | | ONB Gr-I |
| 24 | F | mass nasopharynx | ONB, NHL, Small cell carcinoma | - | | | | | | | - | ++ | | | | ONB Gr-II |
| 43 | F | mass nasopharynx | RMS, Lymphoma, Poorly differentiated ca, ONB | - | | | | - | | + | | | | - | - | Embryonal RMS |
| 35 | M | gluteal region | RMS / Lymphoma | - | | | | - | | + | | | | | | Pleomorphic RMS |
| 1 MONTH | F | adrenal mass | Neuroblastoma | | | | | | | | | + | + | | | Neuroblastoma |
| 5 DAYS | M | adrenal mass | Neuroblastoma | | | | | | | | | + | + | | | Neuroblastoma |
| 40 | F | cervical biopsy | Small (oat) cell ca,poorly differentiated ca,lymphoma | - | | | | + | | | | + | + | | | Small (oat) cell ca |
| 28 | M | cerebellar tumor | Medulloblastoma / lymphoma | - | | | | | | | | + | | | Focal + | Medullo- blastoma |
| 1 year & 6 months | M | right renal mass | Wilms tumor | | | | | | | | | | | | | Wilms tumor |
| 2 | M | right renal mass | Wilms tumor | | | | | | | | | | | | | Wilms tumor |
| 1 | M | liver mass | Hepatoblastoma | | | | | | | | | | | | | Hepato- blastoma |
Discussion
The family of the neoplasms which are generally known as Small Round Cell Tumours (SRCT) has grown in an increasingly complex fashion in recent years. This group of neoplasms is characterized by a high cellularity, small cell sizes and generally diffuse patterns of growth, and it is generally seen in all the age groups. The tumour cells have darkly staining nuclei and scanty cytoplasm; this is seen as an over all blue appearance in the haematoxylin and eosin stained sections, which has led to the alternative term of “small blue cell tumour”.
Generally, the term, ‘SRCT’ has been reserved for the neoplasms which are located in the skeletal system or in the somatic soft tissue and it is usually not applied to other malignant neoplasms of infants and children, that can also be composed predominantly or entirely of small cells, such as Wilm’s tumour, hepatoblastoma or medulloblastoma. Traditionally, the main members of the SRCT group have been malignant lymphoma, Ewing’s sarcoma, rhabdomyosarcoma and adrenal neuroblastoma.
Only two decades ago, the criteria for distinguishing these various entities had become relatively easier, when a combination of topographic, morphologic and ultra structural features were assessed. In the recent past, an increasingly sophisticated array of immunohistochemical and chromosomal markers have proven to be useful in classifying these aggressive lesions.
It is necessary to identify the SRCTs specifically, because they are genetically and biologically different. While a careful examination of the haematoxylin and eosin sections of the well sampled tumours and the availability of the clinical radiological findings help in providing a differential diagnosis, it is important to employ a panel of antibodies (rather than a single marker) and to integrate all these available evidences for a conclusive diagnosis.
As the interpretation of the immunohistochemical results will strongly influence the final diagnosis in a significant amount of cases, a few general rules must be followed. The technical quality and the reproducibility must be controlled. Immunohistochemistry should always be used as an adjunct to the morphology, to avoid an erroneous diagnosis. The antibodies must always be chosen, based on the histological differential diagnosis. The wide “random” panels can be misleading. A correct panel of antibodies should be used, because of the lack of sensitivity or specificity of the markers, and of the frequent “aberrant” immunoreactivities. The use of a single immunostain can also lead to misdiagnoses.
In our study, a total of 43 cases of SRCTs were identified and studied. We noted that 44% of the cases had occurred in the 15-45 years age group, with a male preponderance (74% were males and 26% were female patients). In 40 cases, a panel of monoclonal antibodies was utilised, and we were able to come to a final diagnosis for all these cases. The two cases of Wilm’s tumour which presented as renal masses in an 18months child and in a 2 year old child on microscopy, showed blastemal and also focal epithelial differentiations. Similarly, the hepatoblastoma case had presented in a one year old child with a liver mass, with elevated serum Alpha Fetoprotein (AFP) levels and the haematoxylin and eosin stains had shown the epithelial type of foetal and embryonal liver cells. Their diagnoses were obvious from the microscopic patterns of the arrangement of the tumour cells in the haematoxylin and eosin stains and also from their classical clinical presentations. Hence, immunophenotypic markers were not necessary for the Wilm’s tumour and the hepatoblastoma cases.
We had 12 different cases of Small Round – Cell Tumours (SRCTs). Among these, Non – Hodgkin’s lymphoma was the predominant one, which accounted for 44.2% of the total cases. The others included Ewing’s/Primitive Neuroectodermal Tumour (PNET) (14%), atypical carcinoid (7%), olfactory neuroblastoma (7%), rhabdomyosarcoma (4.7%), neuroblastoma (4.7%), monomorphic synovial sarcoma (4.7%), Wilm’s tumour (4.7%), small cell osteosarcoma (2.3%), small (oat) cell carcinoma (2.3%), hepatoblastoma (2.3%) and medulloblastoma (2.3%).
As shown in Dabbs’ diagnostic immunohistochemistry, Non – Hodgkin’s lymphoma shows positivity for CD45 (95%), the B – cell type (97%) and the T – cell type (89%). In addition, CD3 and CD20 are very specific for the T cells and the B cells respectively [1]. In this study, the initial differentials in 19 biopsies from various organs, based on the haematoxylin and eosin stained sections were Non – Hodgkins’s lymphoma, poorly differentiated carcinoma and Ewing’s sarcoma/Primitive Neuroectodermal Tumour (PNET), which on immunophenotyping, had shown strong positivities for CD45(19/19), CD20 (12/19) and CD3 (7/19), which were finally diagnosed as B – cell and T – cell lymphomas respectively.
The studies which were done by Folpe AL et al., had shown the expression of CD99 in Ewing’s/PNET (100%), [2] T cell lymphoblastic lymphoma (100%) and B cell lymphoblastic lymphoma(60%) [3].
Similar studies which were done by Tadashi Terada showed synaptophysin positivity (85%) and chromogranin positivity (62%) in the carcinoids [4]. In the present study, all the three cases was positive for these neuroendocrine markers. David Dabbs’ diagnostic immunohistochemistry showed the tumour cell positivity for synaptophysin in olfactory neuroblastoma (100%) [5]. The same was noted in all the 3 cases of olfactory neuroblastomas in the present study.
In our study, it was seen that CD99 was expressed in all the cases (100%) of Ewing’s/Primitive Neuroectodermal Tumour (PNET) in a membranous fashion. However, it was also expressed in other tumours. One case of Non-Hodgkin’s lymphoma showed a focal positivity for CD99 in conjunction with the CD45 positivity. One case of small cell osteosarcoma and one monomorphic synovial sarcoma also expressed CD99.
Immunohistochemistry may be inapplicable for the undifferentiated or the poorly differentiated tumours, although in our study, we did not face such a difficulty and were able to reach a final diagnosis for all the 40 cases. The reactivities to the antibodies can vary, depending on the preparation of the specimen, the antibody which is used, and the degree of the tumour differentiation.
The two cases of Wilm’s tumour did not show any features of anaplasia. In case of the neuroblastomas, one had a favourable histopathology and the other had an unfavourable one.
Conclusion
The SRCTs are a heterogeneous group of malignant neoplasms. IHC represents a rapid and a cost-effective tool that can provide a clear distinction among the various tumour types. Its purpose is to categorise the patients in order to ensure an appropriate and a specific treatment, as well as to identify the tumours which are at a higher risk of recurrence and fatal outcomes. Because IHC can provide such important information, it must be performed at a high standard, so that the results are meaningful and reproducible. In recent years, a better understanding of the molecular genetic studies of these tumours allows a molecular testing as a further valuable tool for arriving at a definitive diagnosis in the questionable cases.