Breast cancer is the most commonly occurring female cancer and the leading cause of cancer deaths worldwide. Breast cancer is a heterogeneous disease and it encompasses a variety of entities with distinct morphological appearances and clinical behaviours. In recent years, it has become evident that this diversity is the result of genetic alterations [1]. The analysis of gene expression data has suggested that breast cancers can be divided into molecular subtypes which have distinct clinical features, with markedly differing prognoses and clinical outcomes [2]. These subtypes consist of two ER positive types (Luminal A and Luminal B), and three ER negative types (HER-2 expressing, basal-like and normal breast-like).
Triple-negative breast cancers are a group of primary breast cancers which lack the expressions of the oestrogen receptor (ER), the progesterone receptor (PR) and HER-2. Although the triple-negative phenotype has been considered as sufficient to identify the ‘basal-like’ tumours, increasing evidence has shown that the terms ‘basal-like’ and ‘triple-negative’ are not synonymous [1].
The basal-like tumours typically show low expressions of ER and HER-2, and they exhibit high expressions of the genes which are characteristic of the basal epithelial cell layer. The basal-like tumours have been associated with poor clinical outcomes [2].
Neilsen et al., proposed an immunohistochemical (IHC) panel which comprised of ER, EGFR, HER-2 and CK 5/6, which could identify the breast carcinomas with a basal – like phenotype which was the same as was defined by cDNA microarrays [3,4].
This study has illustrated the clinico pathological characteristics and the immunohistochemical expressions of 50 female patients with triple-negative breast cancer.
Material and Methods
The study sample: A total 50 female patients with triple-negative primary invasive breast carcinoma were selected, based on the ER, PR and the HER-2 negativities and they underwent modified radical mastectomies. The samples were received in the Department of Pathology of the K S Hegde Medical Academy, Mangalore, India. Detailed histories were taken from each of them and clinical examinations were done after taking their informed consents. The study was approved by the institutional ethical committee. The specimens were processed and fixed in 10% formalin. They were examined grossly according to the standard guidelines [5]. 4 – 5μm thick, formalin fixed and paraffin embedded sections were stained with the haematoxylin and eosin stain. The tumours were classified and graded according to the WHO and the Nottingham modification of the Scarff – Bloom – Richardson system. The pathological variables were evaluated. The tissue sections were used for all the immunohistochemical analyses (ER, PR and HER-2). The antibody clones which were used were: those for the oestrogen receptor (ID5 Biogenex), the progesterone receptor (PR 88 Biogenex) and HER – 2(EP1045Y Biogenex). The ER and PR scores were based on the proportions and the intensities of the stained nuclei [5,6]. The HER – 2 score was based on the intensities and the proportions of the cells which showed membrane staining [5,7]. 50 cases with the triple negative phenotype were assessed for the expressions of EGFR, CK 5/6 and Ki-67.
The antibody clones which were used were: EGFR (18C9NOVO, DAKO), CK 5/6 (D5/16B4, DAKO) and Ki – 67 (MIB – 1, DAKO). Cytokeratin 5/6 was scored as positive if any weak or strong cytoplasmic and /or membranous invasive carcinoma cell staining was observed [8].
EGFR was scored as positive if more than 1% of the tumour cells showed membrane reactivities which were above the background [8]. The Ki – 67 positivity was quantified as the percentage of the positive nuclear staining of the tumour cells. 10% was the cut off for the active proliferation [9].
Statistical Analysis
The data were entered and analysed by using SPSS. The frequencies and the percentages of all the variables were computed. The Chi – square (χ2) test was used to compare the association of the expressions of EGFR and CK5/6 and the macroscopic and the microscopic characteristics of the tumours. The results were considered as statistically significant if the p value was <0.05.
Results
A total of 50 cases of infiltrating breast carcinomas of the triple negative phenotype were included. The tumour characteristics of the triple negative breast tumours have been presented in [Table/Fig-1].
Clinicopathological characteristics of Triple-negative breast cancer (ER negative, PR negative and HER-2 negative)
| Clinicopathological variables | Frequency (percentage) N=50 |
| Age (years) |
| Mean | 46.8 |
| Median | 46.0 |
| Tumor size (cm) |
| 0-2 | 5 (10) |
| 2.1 – 5 | 34 (68) |
| >5 | 11 (22) |
| Histopathological diagnosis |
| IDC NST | 44 (88) |
| Medullary carcinoma | 5 (10) |
| Metaplastic carcinoma | 1 (2) |
| Tumor necrosis |
| Present | 28 (56) |
| Absent | 22 (44) |
| DCIS |
| Present | 21 (42) |
| Absent | 29 (58) |
| Tumor grade |
| I | 0 |
| II | 12 (24) |
| III | 38 (76) |
| Pushing margin |
| Present | 28 (56) |
| Absent | 22 (44) |
| Lymphocytic infiltrate |
| Present | 22 (44) |
| Absent | 28 (56) |
| LV invasion |
| Present | 10 (20) |
| Absent | 40 (80) |
| Lymph node metasatases |
| Absent | 13 (26) |
| 1-3 | 20 (40) |
| 4 and above | 17 (34) |
| Ki-67 |
| >10% | 32 (64) |
| <10% | 18 (36) |
Abbreviations – IDC NST – Infiltrating ductal carcinoma no special type DCIS – Ductal carcinoma in situ LV invasion – lymphovascular invasion.
The mean and the median ages were 46.8 and 46.0 years respectively (range 37 – 58 years). Infiltrating ductal carcinoma of no special type was the predominant histopathological type (44 of 50, 88%). In a majority of the patients, the tumour size was between 2.1 to 5cm (34 of 50, 68%). The histopathological evaluation showed a large proportion of patients with poorly differentiated high grade tumours (38 of the 50 patients, 76%), tumour necrosis in 56% patients (28 of the 50 patients) and ductal carcinoma in situ in 42% patients (21 of the 50 patients). Pushing tumour margins, lymphocytic infiltrates and lymphovascular invasions were observed in 28 of the 50 patients, (56%) in 22 of the 50 patients, (44%) and in 10 of the 50 patients (20%) respectively. Lymph node metastases were noted in 37 of the 50 patients, (74% tumours). More than a 10% nuclear staining for Ki-67 was seen in 32 of the 50 patients (64% tumours) [Table/Fig-2].
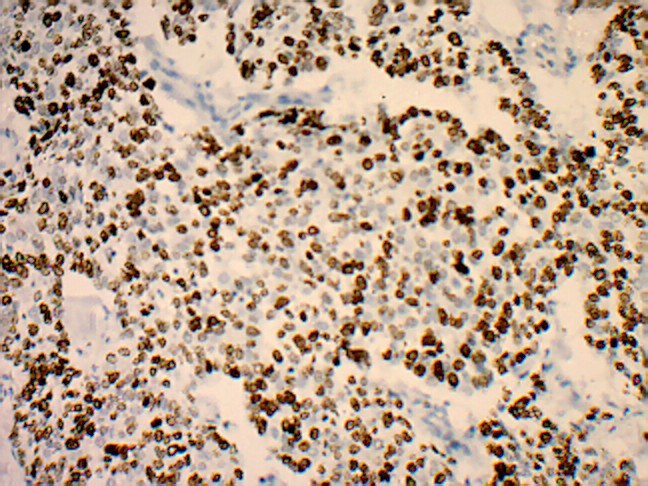
80% nuclear staining for proliferation marker Ki-67 (original magnification x 400)
The frequency of the basal marker expression was assessed. Out of the 50 triple negative breast cancers, 37 (74%) are positive for CK 5/6 and or /EGFR. 13 (26%) cases were neither positive for EGFR nor for CK 5/6 [Table/Fig-3] [Table/Fig-4 and 5].
Summary of immunohistochemical results for basal markers
| Immunohistochemical panel | Frequency (percentage) N = 50 |
|---|
| CK 5/6 + EGFR + | 15(30) |
| CK 5/6 + EGFR - | 5 (10) |
| CK 5/6 – EGFR + | 17(24) |
| CK 5/6 – EGFR - | 13(26) |
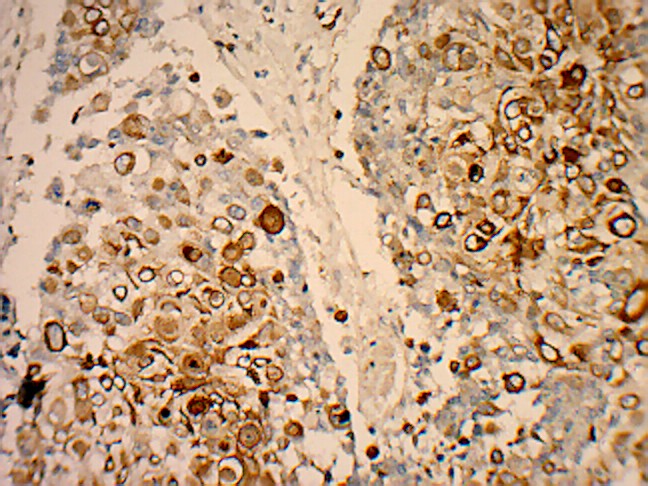
Positive immunohistochemical staining for CK5/6 (original magnification x 400)
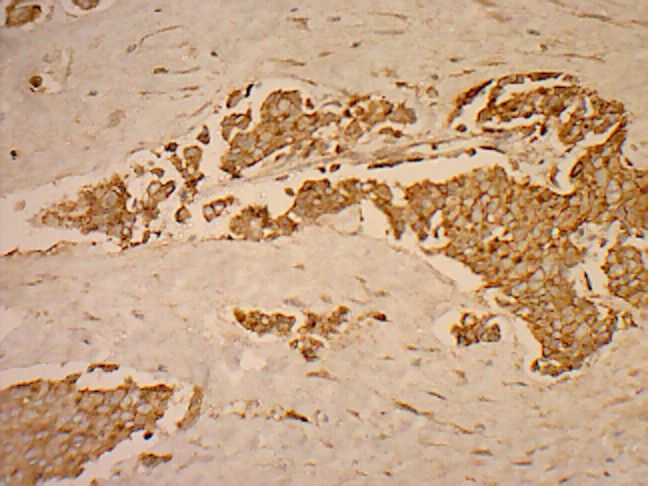
Positive immunohistochemical staining for EGFR (original magnification x 200)
We studied the association between the basal marker expression and the clinicopathological prognostic parameters [Table/Fig-6, 7 and 8]. A significant positive association was observed between the tumour necrosis and the basal marker expression. A majority of the tumours were of the histological grade 3 (28 of the 37 patients, 75.6%), with lymph node metastases in 25 of the 37 patients and with high proliferation rates (24 of the 37 patients, 64.8%).
Association between basal marker expression and prognostic parameters (age and tumor size)
| Prognostic parameters | CK 5/6 and /or EGFR | EGFR | CK 5/6 | p value |
|---|
| positive | negative | positive | negative | positive | negative |
| Age (years) |
| <40 | 5 | 1 | 3 | 3 | 2 | 4 | >0.05 |
| 41 – 50 | 22 | 9 | 21 | 10 | 13 | 18 |
| >50 | 10 | 3 | 8 | 5 | 5 | 8 |
| Tumor size (cm) |
| 0 – 2 | 3 | 2 | 2 | 3 | 3 | 2 | >0.05 |
| >2 | 34 | 11 | 15 | 30 | 29 | 16 |
Association between basal marker expression and prognostic parameters (necrosis, tumor grade, pushing margin, lymphocytic infiltrate and Ki-67 expression)
| Prognostic parameters | CK 5/6 and /or EGFR | EGFR | CK 5/6 | p value |
|---|
| positive | negative | positive | negative | positive | negative | |
|---|
| Necrosis |
| Present | 21 | 1 | 18 | 4 | 16 | 6 | 0.002* |
| Absent | 16 | 12 | 14 | 14 | 4 | 24 |
| Tumor grade |
| I | 0 | 0 | 0 | 0 | 0 | 0 | >0.05 |
| II | 9 | 3 | 9 | 2 | 4 | 8 |
| III | 28 | 10 | 23 | 15 | 16 | 22 |
| Pushing margin |
| Present | 21 | 7 | 18 | 10 | 12 | 16 | >0.05 |
| Absent | 16 | 6 | 10 | 8 | 8 | 14 |
| Lymphocytic infiltrate |
| Present | 17 | 5 | 12 | 10 | 12 | 10 | >0.05 |
| Absent | 20 | 8 | 20 | 8 | 8 | 20 |
| Ki – 67 |
| >10% | 24 | 8 | 18 | 12 | 14 | 16 | >0.05 |
| <10% | 13 | 5 | 14 | 6 | 6 | 14 |
*p value significant
Association between basal marker expression and prognostic parameters (histopathological type and lymph node metastases)
| Prognostic parameters | CK 5/6 and /EGFR | EGFR | CK 5/6 | p value |
|---|
| positive | negative | positive | negative | positive | negative | |
|---|
| Histopathological type |
| IDC NST | 31 | 13 | 29 | 15 | 16 | 28 | >0.05 |
| Medullary | 5 | 0 | 2 | 3 | 4 | 1 |
| Metaplastic | 1 | 0 | 1 | 0 | 0 | 1 |
| Lymph node metastases |
| Absent | 12 | 1 | 10 | 3 | 8 | 5 | >0.05 |
| 1-3 | 14 | 6 | 12 | 8 | 5 | 15 |
| 4 and above | 11 | 6 | 10 | 7 | 7 | 10 |
Discussion
The triple – negative phenotype of breast cancer has been reported to have different incidences amongst different ethnic groups, with a lower disease-free survival, a higher predisposition to form visceral metastases and poorer outcomes as compared to the other subtypes of breast cancer [10].
In the present study, the triple-negative breast cancer correlated significantly with a younger age at diagnosis and a higher histological grade, which correlated with the results which were obtained by Dent et al 2007, Rakha et al., Reis-Filho et al., and Thike et al., [11–14]. A majority of the triple – negative breast cancers are high grade invasive ductal carcinomas of no special type, and the remaining are medullary carcinomas and metaplastic carcinomas, thus suggesting that the triple negativity can occur in all the histological subtypes of breast cancers, with possible implications on their pathogeneses, progressions and prognoses [13,14].
In the current study, necrosis was an important morphological factor in the triple negative breast cancer. We observed necrosis (focal/comedo) in a large number of tumours. Thike et al., observed frequent necrosis in their study [14]. There was no positive association with the pushing margins, the presence of lymphocytic infiltrates and the lymphovascular invasion. The presence of intratumoural lymphocytes is an independent prognostic indicator for an improved patient survival in TNBC [15,16].
There are conflicting results on the prevalence of lymph node metastases at the time of the diagnosis in the patients with TNBC. In our study, the TNBCs included higher rates of node –positive cases, which was in aggreement with the findings of Rakha et al., and Carey et al., [12,17]. Some series had results which were different from ours [18]. Unlike the findings of Thike et al., who found a relationship between the tumour size and the nodal metastasis, our cases showed a decreasing incidence of the axillary lymph node metastasis with the enlarging tumour sizes. Similar results were obtained by Dent et al., [11].
Ki – 67, a proliferation marker, is an independent predictive and a prognostic factor. A majority of the TNBCs in our study showed more than a 10% nuclear staining for Ki – 67. The cases with high Ki – 67 expressions respond better to the chemotherapy, with a poor prognosis [9]. TNBCs are associated with higher expressions of Ki – 67 and a poor survival [9].
We examined the expressions of EGFR and CK5/6 by immunohistochemistry. In a majority of the triple negative breast tumours, we found the expression of at least of one of the markers, EGFR and /or CK 5/6. (74%) Our results were consistent with those of previous studies [12,19, 20]. This suggested that the TNBCs were heterogeneous in nature. The individual basal marker, namely EGFR, was expressed in more than half of the triple-negative tumours(64%), which was consistent with the earlier reports [3,21] and CK 5/6 was expressed by more than quarter of the triple negative tumours (40%), which was less as compared to that in earlier studies [3,21]. Our observations imply a large overlap between the “triple negativity” and the “basalness”. Similar findings were reported by Rakha et al., and Choccalingham et al., [8,12]. The term, “core basal phenotype” has been used to define a clinically relevant subtype of breast cancer – that is, a tumour that has a triple negative status but which also expres-ses CK5/6, EGFR, or both and which may have a worse
outcome than the breast cancers that are negative for all these markers [22].
A large number of patients who showed the basal marker expression were in the age group of 41 to 50 years, with tumour sizes of more than 2cm. A significant positive association was noted between the presence of the tumour necrosis and the basal marker expression, which has not yet been documented till date [23]. A majority of the tumours with the basal marker expression were poorly differentiated high grade tumours with pushing margins, tumour lymphocytic infiltrates, lymph node metastases and high proliferation rates, which was consistent with the findings of earlier studies [12,14]. Studies have shown that the basal-like subtype was associated with poor clinical outcomes [2].
Chemotherapy is currently the mainstay of the systemic medical treatment for TNBC. 17-58% of the patients with triple-negative breast cancers have been shown to have a pathological complete response after the anthracycline + taxane based neoadjuvant chemotherapy [1]. The overexpression of EGFR is more common in the TNBCs than in other subtypes of breast cancer, and the use of the monoclonal antibody, cetuximab, which is targeted against EGFR, is being further studied in combination with carboplatin [22].
Our study contributes to the growing body of evidence which suggests that the clinical behaviours and the metastatic patterns of the basal-like breast cancers in a triple-negative subgroup are different from those of the non basal-like breast carcinomas.
In conclusion, the term, ‘Triple-Negative Breast Cancer’ encompasses a heterogeneous group of tumours that possess distinctive pathological and clinical features. Although, a significant overlap was observed between the triple-negative breast cancer and basal-like breast carcinoma, the “triple negativity” should not be used as a surrogate marker for the basal-like breast cancers. By adding, CK 5/6 and EGFR as the positive markers to the triple negative phenotype, a significantly worse outcome group can be identified among the triple-negative cases. A majority of the“triple negative” patients have tumours of the basal subtype with EGFR expression. The basal phenotypes have more aggressive pathological features than the non-basal phenotypes. Elucidating the molecular basis behind the necrosis in basal-like breast cancers could lead to the discovery of therapeutic agents. This subgroup of breast carcinomas could potentially benefit the most from the novel EGFR-targeted therapeutic strategies [24].
Abbreviations – IDC NST – Infiltrating ductal carcinoma no special type DCIS – Ductal carcinoma in situ LV invasion – lymphovascular invasion.
*p value significant