Adenomyosis, one of the most common debilitating diseases which affects women of the reproductive age group, was first described by Rokitansky in 1860 [1]. It refers to the haphazard presence of the endometrial glands and the stroma, deep within the myometrium, along with the changes in the response to the monthly physiological hormonal changes. The prerequisite for adenomyosis may be triggered or facilitated by either a ‘weakness of the smooth muscle tissue or an increased intrauterine pressure, or both’. The cause of the adenomyosis is unknown, although it has been associated with any sort of uterine trauma that may break the barrier between the endometrium and the myometrium, such as a caesarean section, a tubal ligation, a pregnancy termination and a pregnancy. Despite the fast development of science and technology, no single theory can still explain the pathophysiology of endometriosis [2].
It is technically too difficult to investigate the aetiology and the pathogenesis of adenomyosis in human models. Animal models were devised for that reason. Mori succeeded in developing adenomyosis by doing an intrauterine hypophysis transplantation and by causing hyperprolactinaemia [2]. In the 1980s adenomyosis was developed experimentally in animals by using diethylstilbesterol and dopamine antagonists [3].
Fluoxetine is a selective serotonin reuptake inhibitor (SSRIs) which was developed in the late 1970s, with the idea of an effective treatment for the patients who were suffering from depression. Fluoxetine affects the 5HT c/2 receptors, thus inhibiting the serotonin reuptake and causing an increase in the prolactin secretion [1]. The adenomyosis inducing effect of prolactin can be explained, as was defined by Mori as follows. Ultrastructural studies have shown a close relationship between the prolactin and myometrial degeneration, which ultimately leads to adenomyosis [4].
A prospective interventional study was devised, to investigate whether the fluoxetine induced hyperprolactensemia could induce adenomyosis in rats. For a clinical correlation, a comparison between the mean prolactin levels of the adenomyosis patients and those of their age and sex matched otherwise healthy controls was done. The female patients who were receiving fluoxetine for more than 3 months were also subjected to the serum prolactin level measurement.
Material and Methods
Prior to the initiation of the study, necessary permission was obtained separately for both the clinical as well as the animal studies from the Institutional Ethics Committee and the Institutional Animal Ethics Committee.
The animal experimentation
18 female Wistar Albino rats (200-240gms) were procured from the institutional animal house. They were kept in standard plastic rat cages and fed with a standard rat food which was in pellet form (which was bought from Hindustan Animal Feeds) and tap water ad libitum. The rooms were equipped with lighting, conditioning, moisture and heat controls. The maintenance of the animals as well all the procedures of the experiment were as per the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines. The animals were adapted to their new surroundings for three days before the initiation of the experiment.
The 18 rats were divided into three groups, namely, group I, the vehicle treated control group (0.9% normal saline), group II, which was treated with oral Fluoxetine 4mg/kg (Afzot tab 20mg, Pfizer, dissolved in 0.9% normal saline 20mg/5ml ) and group III, which was treated with Fluoxetine 8mg/kg (Afzot tab 20mg, Pfizer, dissolved in 0.9% normal saline 20mg/5ml) [1].
The fluoxetine hydrochloride was dissolved in 0.9% normal saline. This solution was prepared in such a way that each ml contained 4 mg of fluoxetine hydrochloride. The rats were fed with this solution by using a BCG syringe and a rat feeding cannula for 100 days. Blood samples were collected by cutting the tips of the tails of the rats with surgical blades at the initiation of the study i.e., before the administration of any drug, on the 50th day and at the end of the study i.e., at the end of 100 days, for the estimation of prolactin. The prolactin estimation was done by using the Prolactin R Rat ELISA kit (ab3351).
The bleeding was stopped by using finger pressure over the cut area. The collected blood samples were allowed to coagulate at room temperature. The samples were then centrifuged at 3000 rpm for 20 minutes, to extract the sera, which were collected with the help of a micropipette and they were stored at -200C.
After 100 days, all the rats of all the three groups were sacrificed humanely by stunning and cervical decapacitations and their uteri were removed and kept in a 10% formaldehyde solution for 24 hrs. Later, they were embedded in paraffin and 7 micrometre (μm) sections were taken. These sections were then stained with haematoxylene-eosin by the standard method. The paired t test was used for the comparison of the serum prolactin levels before and after the fluoxetine administration.
The clinical part
The patients (who were between 18 to 45 years) who were newly diagnosed as having adenomyosis by the gynaecologists at the outpatients department by clinical as well imaging techniques were selected for screening. Then, every patient was individually assessed to exclude or include her in the study as per the exclusion criteria. The inclusion criteria were age between 18 and 45 years and not taking the following drugs, namely, anxiolytics, diuretics, hormonal contraceptives and neuroleptics. The exclusion criteria were lactating mothers and those who were suffering from liver, kidney or any hormonal disorders. Informed written consents (approved by the IEC) were obtained from the participants after discussing different aspects of the study with them in a vernacular language.
All the patients who had attended the gynaecology OPD during the study period and had also met the inclusion and exclusion criteria for the present study were enrolled.
Fifteen patients were thus selected. The serum prolactin level of each patient was measured and it was compared with that of each of the 15 age and sex matched healthy females.
Again, 20 female patients (who had been just prescribed fluoxetine for any indication) were selected from the Psychiatry OPD for the measurement of their serum prolactin levels, considering the inclusion criteria, namely, who were aged between 18 and 45 years, those who were taking only fluoxetine and no other antipsychotic or antidepressant drugs, and those who were willing to take part in the study and the exclusion criteria, namely, the patients who were taking different antipsychotic drugs besides fluoxetine, lactating mothers and those who were known to suffer from liver, kidney or hormonal diseases. The serum prolactin levels at the baseline (day 0) as well as those at the end of the study (at the end of 3 months i.e., 90 days) were measured and compared.
The unpaired “t” test was used for the comparison of the serum prolactin levels between the adenomyosis patients and the age and sex matched healthy controls, whereas the paired “t” test was used for the comparison of the serum prolactin levels before and after the administration of fluoxetine to the patients who were selected from the Psychiatry OPD.
Result and Analysis
The animal experimentation
The serum prolactin levels of the rats of both the control and the test groups (the fluoxetine treated groups) were measured at the initiation as well as at the end of the study, along with the histopathological examinations of the uteri of the rats of both the groups. Here, we found that the serum prolactin levels had risen with the higher doses of fluoxetine in a dose dependent manner [Table/Fig-1].
A graphical representation of the serum prolactin levels
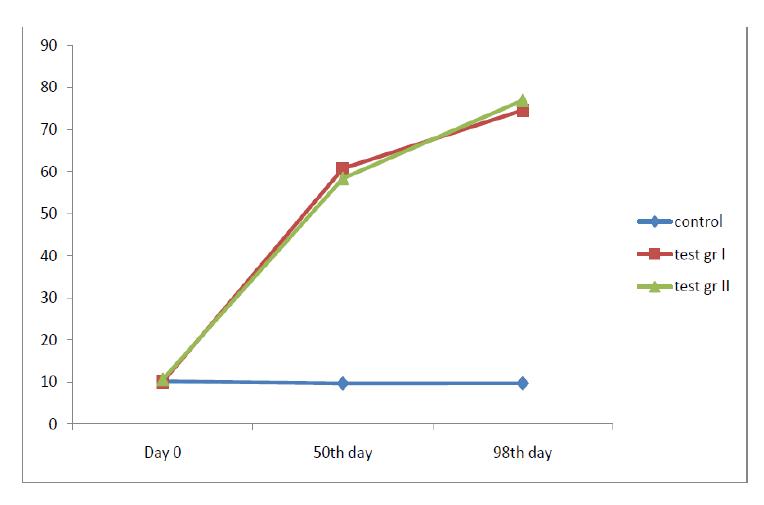
At the baseline, there was no significant variability in the serum prolactin levels among the two- the test as well as the control groups [Table/Fig-2]. The serum prolactin levels which were measured on the 50th day in all three groups, namely, the control group (vehicle treated), the test group I (Fluoxetine treated 4mg/kg) and the test group II (Fluoxetine treated 8mg/kg), showed a rise in the serum prolactin levels in both the test groups [Table/Fig-3].
Serum prolactin levels (prior to any drug administration)
| Groups | Serum prolactin levels (ng/ml) (normal range of prolactin is between nondetectable level to <18ng/ml) |
|---|
| Mean±SD | Range |
|---|
| Group I (Control group) | 10.17±1.11 | 9-11.2 |
| Group II (treated with oral Fluoxetine 4mg/kg) | 10.093333±1.5 | 8.98-11.8 |
| Group III (treated with oral Fluoxetine 8mg/kg) | 10.66667±1.09 | 9.5-11.65 |
Serum prolactin level at the 50th day of the study
| Groups | Serum prolactin levels (ng/ml) (normal range of prolactin is between nondetectable level to <18ng/ml) |
|---|
| Mean±SD | Range |
|---|
| Group I (Control group) | 9.66±0.58 | 9 -10 |
| Group II (treated with oral Fluoxetine 4mg/kg) | 60.67667±4.08 | 57.67-65.4 |
| Group III (treated with oral Fluoxetine 8mg/kg) | 58.41±2.75 | 55.25-60.23 |
Serum prolactin levels at 100th day
| Groups | Serum prolactin levels (ng/ml) (normal range of prolactin is between nondetectable level to <18ng/ml) |
|---|
| Mean±SD | Range |
|---|
| Group I (Control group) | 9.71±1.25 | 8.5-11 |
| Group II (treated with oral Fluoxetine 4mg/kg) | 74.61±3.90 | 70.5-78.35 |
| Group III (treated with oral Fluoxetine 8mg/kg) | 76.97±2.13 | 73.78-79.56 |
Serum prolactin levels expressed in mean and standard deviation (SD) of all the three groups at three time interval (day 0, day 50 and day 100)
| Group I | Group II | Group III |
|---|
| Day 0 |
Day 50th |
Day 100th | Day 0 |
Day 50th |
Day 100th | Day 0 |
Day 50th |
Day 100th |
|---|
| Mean | 10.16 | 9.66 | 9.71 | 10.09 | 60.76 | 74.61 | 10.66 | 58.41 | 76.96 |
| SD | 1.11 | 0.58 | 1.25 | 1.5 | 4.08 | 3.93 | 1.08 | 2.74 | 2.12 |
Serum prolactin levels (ng/ml) in control as well as adenomyosis group
| Serum prolactin level (ng/ml) normal range of prolactin is between 2 and 29 ng/ml |
|---|
| S/L no | Control group | Adenomyosis group |
|---|
| 1 | 17.54 | 16 |
| 2 | 12.32 | 19 |
| 3 | 10 | 22 |
| 4 | 10.65 | 20.5 |
| 5 | 11.23 | 17 |
| 6 | 12.35 | 15 |
| 7 | 15.4 | 27.5 |
| 8 | 13 | 18 |
| 9 | 12.43 | 20 |
| 10 | 12 | 19 |
| 11 | 15.8 | 21.45 |
| 12 | 16.44 | 20 |
| 13 | 13.76 | 18 |
| 14 | 18.43 | 25 |
| 15 | 16 | 15 |
The serum prolactin levels which were measured on the 100th day in all three groups, namely, the control group (the vehicle treated group), Group II ( which was treated with oral Fluoxetine 4mg/kg) and Group III (which was treated with oral Fluoxetine 8mg/kg), showed a rise in the serum prolactn levels in both the test groups [Table/Fig-4].
Comparison between serum prolactin levels on day 0 and at the end of three months of patients taking fluoxetine
| Patient’s Id no | Prolactin level (ng/ml) normal range of prolactin is between 2 and 29 ng/ml |
|---|
| Baseline |
At the end of 3rd month
|
|---|
| 1 | 17 | 16 |
| 2 | 14 | 21.54 |
| 3 | 18.76 | 21 |
| 4 | 15.55 | 18.9 |
| 5 | 17.98 | 18 |
| 6 | 17.45 | 20 |
| 7 | 13 | 16.5 |
| 8 | 14.45 | 21 |
| 9 | 12.43 | 19 |
| 10 | 15.54 | 19.65 |
| 11 | 15.8 | 19.4 |
| 12 | 15.4 | 16.5 |
| 13 | 13 | 16.75 |
| 14 | 18.43 | 18.65 |
| 15 | 19 | 20 |
| 16 | 14 | 18 |
| 17 | 16.6 | 17.6 |
| 18 | 15.7 | 18.32 |
| 19 | 17.8 | 19 |
| 20 | 11.4 | 15.45 |
The serum prolactin levels in both the test groups (group II and group III), which were measured on the 50th and the 100th days, were significantly raised in comparison to their respective day 0 values.
In case of the test group II, p was 0.004 when the prolactin levels were compared between day 50 and day 0. A similar comparison which was done between the prolactin levels on day 100 and day 0 showed the p value to be 0.001. A similar comparison in case of the test group III, of the prolactin levels between day 50 and day 0, as well as between day 100 and day 0, yielded p values of 0.001 in both the cases individually. A graphical representation of the serum prolactin levels at day 0, day 50 and day 100 of the rats of the groups I, II and III has been shown in [Table/Fig-5].
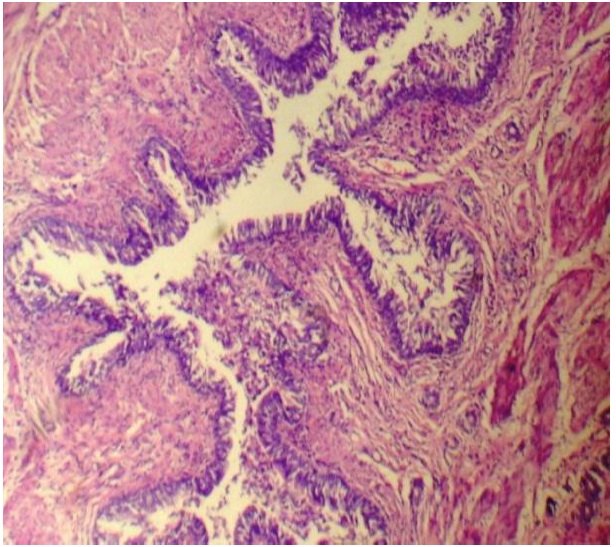
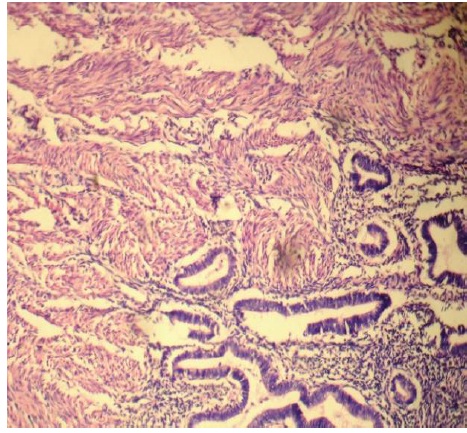
A microscopic examination of uteri of the test group rats (Group II and Group III) showed the following findings:
The horns of the uteri had diameters between 2.5 – 3mm.
The uterine cavity was lined with a single layer of high columnar epithelium. These cells contained giant vacuoles.
A thickening between the inner and the outer muscular layer was detected, which was compared with the controls and a loss of the inner muscular layer was observed.
Endometrial gland islets were seen in the myometrial layers, although they were not seen in the subserosal compartments.
All these findings were found to be consistent with the features of adenomyosis [Table/Fig-6, 7].
The mean prolactin level in the healthy controls was 13.82ng/ml (standard deviation 2.60), whereas that of the adenomyosis patients was 19.56 ng/ml (standard deviation 3.48ng/ml). The comparison between the two groups showed the difference to be significant (p value 0.001)[Table/Fig-8]
Out of the 15 diagnosed patients of adenomyosis, 7 patients (46%) showed prolactin levels which were above the reference range i.e., 19.5 ng/ml for the prolactin kit which was used (highest level 27.5ng/ml).
Similarly, the mean value of serum prolactin prior to any drug therapy was 15.66ng/ml (Standard deviation 2.22) and that at the end of the treatment i.e., at the end of the 3rd month was 18.56 ng/ml (Standard deviation 1.73).
A significant rise in the prolactin levels was seen in the patients who were treated with fluoxetine in comparison to the baseline values (p=0.001). However, only 6 patients (the highest level was 21.54 ng/ml) showed prolactin levels which were above the reference range, which was specified by the prolactin testing kit which was used (the range which was specified was 1.2-19.5 ng/ml), [Table/Fig-9].
Discussion
Adenomyosis is a myometrial lesion which is characterized by the presence of an ectopic endometrium, with hyperplasia of the surrounding myometrium [5]. Only little has been understood about the pathogenesis of adenomyosis, and the clinical studies have hypothesized that adenomyosis results when the endometrial glands invade the myometrial layer. Thus, the surgical disruptions of the endometrial-myometrial border have been shown to increase the risk of adenomyosis in some studies [5, 6]. However, animal models suggest a role of the pituitary hormones, where the elevated levels of both FSH and PRL appear to induce adenomyosis [6, 7].
H & E stained section of uterus showing features of adenomyosis, 400X
H & E stained section of uterus showing features of adenomyosis 100X
Exposure of the murine uterus to an increased PRL, appears to be sufficient to cause histologic adenomyosis [8]. The expression of the uterine PRL receptor messenger RNA is also up-regulated in that model [7]. In addition to this, both the human and animal data suggest a link between the actions of antidepressants in the development of adenomyosis [9, 10].
In vitro studies have demonstrated that PRL is produced by the human uterine tissues and that a functional PRL receptor is present in the uterus, which is capable of acting as a smooth muscle cell mitogen [11].
Fluoxetine, a widely prescribed selective serotonin reuptake inhibitor (SSRI), can raise the serum prolactin levels. Although it may not always cause symptoms or hyperprolactenaemia, it can definitely cause a significant rise in the serum prolactin levels [12].
In our study, we tried to explore the role of the raised serum prolactin in adenomyosis, in rat models, which was induced by the oral fluoxetine administration. On the other hand, in the clinical set up, we compared the serum prolactin levels of the adenomyosis patients with those of the healthy age and sex matched controls.
The significantly raised serum prolactin levels (p<0.001) and the histopathological evidence of adenomyosis in the test group rats indicated the positive role of fluoxetine in the causation of adenomyosis. This was further supported by clinical data, which showed that seven out of the fifteen patients had raised serum prolactin levels in comparison to their healthy counterparts.
Again, in the 20 female patients, taking fluoxetine on a daily basis showed a significant rise in the serum prolactin levels in comparison to the baseline values, which supported the findings of our animal study.
There are several animal studies which have shown raised prolactin levels in the rat models of adenomyosis, which supported our study findings [6, 8, 9].
Although there are no human studies which have shown an association between adenomysosis and the levels of prolactin, it is known that several drugs which include some commonly prescribed psychiatric drugs are capable of raising the prolactin levels [8].
Conclusion
Thus, it can be postulated that high levels of serum prolactin may have some role in adenomyosis. Fluoxetine, which on long term use, can raise the serum prolactin levels. Although it may not be always biochemically or clinically evident, still it may predispose the female patients to adenomyosis.
The small sample size and the lack of follow-up in the adenomyosis patients with regards to studying the serum prolactin levels and the incidence of the occurrence of adenomyosis among the patients who were taking fluoxetine on a long term basis, are the limitations of the study.
Future studies have to be conducted, to find out the incidence of adenomyosis in fluoxetine treated female patients.