Rheumatoid Arthritis (RA) is a systemic, chronic, autoimmune, inflammatory joint disease which had a prevalence of 0.5 - 1% globally in 2012 [1]. However, the prevalence of RA in India was recorded as 0.9% in 2012 [2]. Though it affects all the races, the female population is affected three times more than the male population. The cause of RA is still a mystery. The management of RA includes Nonsteroidal anti-inflammatory Drugs (NSAIDs), Glucocorticoids, Disease- Modifying Anti-Rheumatic Drugs (DMARDs) and Biologic agents. The main role of the NSAIDs is to reduce the joint pain and their chronic use results in numerous side effects, particularly gastrointestinal and cardiovascular side effects. Moreover, the RA patients have increased mortality due to cardiovascular problems [1]. Hence, the scope of the research widens for the newer, effective and the safer alternative medicines.
The n-3 Essential Fatty Acid (EFA) is a ray of hope for a variety of clinical disorders which include RA. Epidemiological studies which were done on Greenland Eskimos suggest about the anti-inflammatory effects of n-3 EFA. The consumption of seafood which is rich in n-3 EFA resulted in a lower incidence of inflammatory and autoimmune diseases such as Psoriasis, Asthma and Type-1 Diabetes, as well as the complete absence of Multiple Sclerosis in humans [3]. Studies have shown that increasing the dietary intake of n-3 EFA through the consumption of fatty fish or fish-oil supplements had reduced the incidence of many chronic diseases that involve inflammatory processes like Cardiovascular diseases, Inflammatory bowel disease (IBD), Cancer, Rheumatoid Arthritis, Psychiatric and Neurodegenerative illnesses [4].
EFA is a component of the plasma membrane of human cells. Dietary EFA leads to the synthesis of Leukotrienes (LTs) and Prostaglandins (PGs) with different pro and anti- inflammatory properties. During the process of their synthesis, there is an EFA competition for the metabolic enzymes. Hence, changing the EFA content in the diet or consuming EFA supplements can alter the types of the LTs and the PGs which are formed [5]. Arachidonic acid (n-6 EFA) leads to the synthesis of highly inflammatory PGs of the 2 series and LTs of the 4 series, which are contrary to Eicosapentaenoic acid (n-3 EFA) and Docosahexaenoic acid (n-3 EFA), which are the precursors of the less inflammatory PGs of the 3 series and LTs of the 5 series. Hence, an increase in the consumption of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) might lead to the lowering of the inflammation [5]. When it is administered as an ethyl ester, EPA appears to form potent anti-inflammatory molecules which are called resolvins [4] and omega-3-oxylipins [6], which may partly explain the anti-inflammatory role of the fish oils. The DHA and EPA may act as direct ligands to a cell surface G-protein receptor, which affects the anti-inflammatory and the insulin sensitization in mice [7]. Moreover, the cod liver oil capsules provide Vitamin A which protects against the Methotrexate therapy induced genetic damage [8] and Vitamin D whose intake is inversely associated with RA [9].
This prompted the present study to evaluate the role of Cod liver oil which contained n-3 EFA in decreasing the daily consumption and probably the side effect profile of Diclofenac Sodium in RA patients.
Materials and Methods
This longitudinal, prospective, open label study was conducted from April to September 2012 in the Departments of Orthopedics and General Medicine at Mahatma Gandhi Medical College and Hospital, Jaipur, India. Thirty RA patients who were aged between 19 to 60 years as defined by the new 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria [10] or the ACR revised functional status class I, II or III [11] or those who were already on medications which included NSAIDs for at least 3 months prior to entering the study were enrolled. Pregnant women and the patients who were taking prednisolone and EFA supplements were excluded. Written informed consents of all the patients and the approval of the Institutional Ethics Committee (IEC) were obtained before the start of the study.
On enrolling the patients, the medical history and the general physical examination, in addition to the epidemiological characteristic profile were noted. Each patient was given five Cod liver oil capsules twice daily for a period of 24 weeks. Each capsule which contained 300 mg of Cod liver oil had EPA-20 mg, DHA-30 mg, Vitamin A-284 IU and Vitamin D-28.4 IU. The patients were assessed for their pain scores by using the Visual Analogue Scale (VAS) (100 mm) at 0, 4, 12, 20 and 24 weeks. The patients who took different NSAIDs daily were switched over to a single brand of Diclofenac Sodium 50 mg tablet. Depending upon the severity of the pain, each patient was asked to take 50 mg Diclofenac Sodium as a single dose, up to a maximum dose of 200 mg per day [12]. The total dose of Diclofenac Sodium which was consumed per day and the average daily requirement at 4, 12, 20 and 24 weeks were recorded in each patient and they were compared. In addition, the ‘Subjective Response’ to pain was assessed in each patient at 4, 12, 20 and 24 weeks.
The Pain Score Assessment
The amount of pain in each RA patient was assessed by employing the VAS pain score [13]. Operationally, VAS is a horizontal line which is 100 mm in length, which is anchored by word descriptors at each end, as has been illustrated in [Table/Fig-1]. The patients mark on the line at the points that they feel represent their perception of their current state of pain. The VAS score is determined by measuring in millimetres from the left hand end of the line to the point that the patient marks [14].
Visual Analogue Scale for pain score
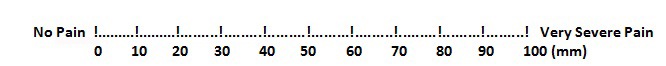
Compliance and Safety Assessment
The compliance was assessed by counting the total number of empty Cod liver oil capsule bottles and the vacant Diclofenac Sodium tablet strips which were submitted to the investigator by the patients during their visits to the hospital. The safety was assessed by enquiring about any adverse event which was encountered during the study period.
Statistical Analysis
The Student’s t-test (paired 2 tailed) was applied for the analysis of the VAS pain score and for evaluation of the reduction in the mean daily dose of Diclofenac Sodium. A probability value of less than 0.05 (p<0.05) was considered to be statistically significant. The results were expressed as Mean ± Standard Deviation. In addition, the results of the ‘Subjective Response’ to pain were expressed as percentage.
Results
Out of the 30 RA patients, only 26 patients completed the study satisfactorily. Four patients withdrew because of a poor compliance. Amongst the 26 patients who completed the study, 34.6% patients were in the age group of 41-50 years and another 34.6% patients were in the age group 51-60 years, while the rest were in different age groups of 19 to 40 years. There were 85% female and 15% male patients. 58% patients of the study belonged to the ACR Class III, while 27% patients were in ACR Class II and the remaining were in ACR Class I. The patients who were included in the study received Cod liver oil capsules concurrently with Diclofenac Sodium for a period of 24 weeks. Any difference in the mean Diclofenac Sodium dose reduction was recorded and compared. Moreover, the patients were assessed and compared for the VAS pain score and for the ‘Subjective Response’ to pain.
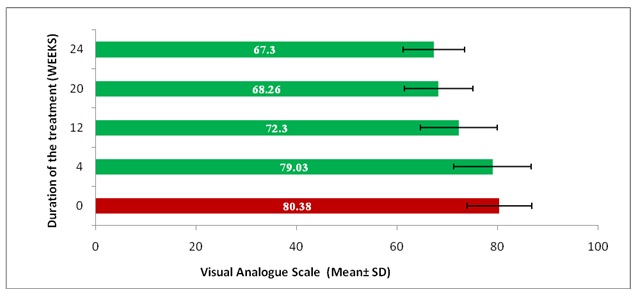
While analyzing the VAS score, it was found that there was a significant difference (p< 0.05) in mean VAS pain score at 12, 20, and 24 weeks (72.30 ± 7.6, 68.26 ± 6.3, 67.30 ± 5.3) respectively, after the treatment as compared to the mean VAS pain score at 0 week (80.38 ± 6.4) before the treatment among the patients. However, a significant but a gradual decrease in the mean VAS pain score was recorded at 12 weeks after the treatment, till 24 weeks, which indicated the effectiveness of the treatment intervention [Table/Fig-2].
Comparison of Mean VAS pain score before (0 week) and after the treatment (4 to 24 weeks)
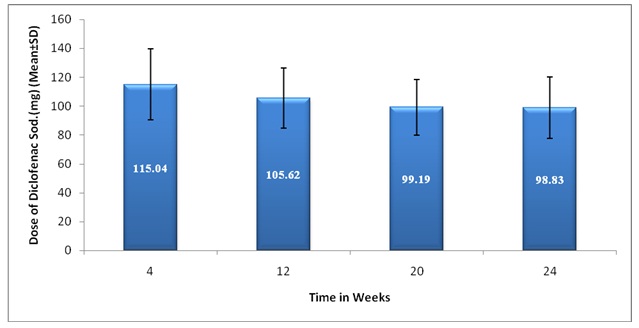
A significant decrease (p<0.05) in mean dose of Diclofenac Sodium consumed per day was noticed in all the patients at 12, 20 and 24 weeks (105.62 ± 20.72, 99.19 ± 19.33, 98.83 ± 22.31) respectively as compared to the mean dose of Diclofenac Sodium consumed per day at 4 week (115.04 ± 24.56) [Table/Fig.3] which suggests the effectiveness of concurrent consumption of Cod liver oil capsules.
Comparison of Mean daily dose of Diclofenac Sodium consumed at 4 to 24 weeks
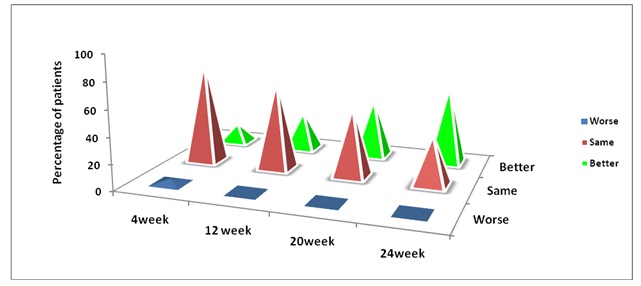
Pain is a symptom with a substantial subjective component, hence; the‘Subjective Response’ to the pain was also assessed in terms of the response to the pain as Worse, Same and Better in each patient at 4, 12, 20 and 24 weeks. This study recorded the ‘Better’ Subjective Response to pain in 61.54% of the patients at the end of the study period as compared to that in 15.38 % patients at week 4, with a significant reduction in the mean daily dose of Diclofenac Sodium when it was taken concomitantly with Cod liver oil capsules [Table/Fig-4].
Percentage of patients showing ‘Subjective Response’ to pain at different visits
Discussion
Rheumatoid Arthritis is a systemic, chronic, autoimmune, inflammatory disease of unknown aetiology, which is characterized by symmetric, peripheral polyarthritis which results in persistent pain and permanent deformity of the affected joints [1]. Different treatment interventions are employed, either to arrest the progression of the disease or to relieve the symptoms. NSAIDs are almost a component of every prescription, in the treatment of the RA patients. NSAIDs are prone to cause a number of side effects, most importantly gastric erosion, peptic ulceration, gastric bleeding and perforation, an increased risk of myocardial infarction and stroke [15].
n-3 EFA, as suggested by previous studies [16, 17], has an anti-inflammatory effect which justifies its role in the concurrent therapy for reducing the daily dose of NSAIDs which are used to control the pain in the RA patients. This study was aimed at evaluating whether the concomitant use of n-3 EFA could reduce the daily dose of Diclofenac Sodium consumed and probably the risk of the side effects which were associated with it in the RA patients.
The patients were cooperative throughout the study period and they were hopeful that they could reduce their persistent pain by a safe intervention. Each RA patient who was included in this study was shifted over to Diclofenac Sodium 50mg per day, up to a maximum dose of 200mg per day, besides receiving five Cod liver oil capsules twice a day for a period of 24 weeks.
Each capsule which contained 300 mg of Cod liver oil had EPA-20 mg, DHA-30 mg, Vitamin A-284 IU and Vitamin D-28.4 IU. Hence, all the patients were given EPA-200 mg, DHA-300 mg, Vitamin A-2840 IU and Vitamin D-284 IU per day i.e. 500mg of n-3 EFA (EPA-200 mg + DHA-300 mg), 2840 IU of Vitamin A and 284 IU of Vitamin D per day for a period of 24 weeks. In the study, we tried to focus on the safe interventions that could be beneficial for the RA patients. The most common cause of the increased mortality in the RA patients was Ischaemic Heart Disease [1].
The consumption of 400- 500 mg per day of n-3 EFA was associated with a reduced mortality which was caused by Coronary Heart Diseases [18]. The intake of 300-600 mg per day of DHA and EPA meet the recommendations for the primary prevention of Coronary Heart Diseases [19]. The dietary recommendations for EPA and DHA, based on the Cardiovascular Disease risk considerations for European adults are between 250 and 500 mg per day [20].
Epidemiological and experimental studies have suggested that the intake of 500 mg per day of EPA + DHA may prevent the development and the progression of Heart Failure [21]. Vitamin A and D supplementations were also considered and they were used as a component of the Cod liver oil formulation, as Vitamin A protects against the Methotrexate therapy induced genetic damage 8 and as a Vitamin D deficiency is highly prevalent in the RA patients [22].
The U. S. Food and Drug Administration recommends that the total dietary intake of n–3 fatty acids should not exceed 3 grams per day, with no more than 2 grams per day from nutritional supplements [23]. The daily tolerable upper intake level for Vitamin A is 3000 μg or 10,000 IU [24] and for Vitamin D, it is 4000 IU [25] for the ages which are above 19 years. Thus, the doses of n-3 EFA, Vitamin A and vitamin D which were used in this study were respectively at the lower end of the daily tolerable upper intake level. Hence, the doses of the components of the cod liver oil capsules which were used in this study automatically reduced the propensity of the toxicity which was related to it.
The mean VAS pain score, the mean dose of Diclofenac Sodium which was consumed per day and the ‘Subjective Response’ to pain in each patient was evaluated at different weeks of the treatment protocol. While assessing the mean pain score by VAS, a significant difference (p<0.05) was recorded at different weeks viz. 0, 4, 12, 20 and 24 weeks before and after the treatment [Table/Fig-2].
The mean VAS pain score which was noted before the treatment at 0 week was 80.38 ± 6.4, which reduced to 67.30 ± 5.3 after the treatment at 24 weeks. This effect of the gradual reduction in the pain could be due to the anti-inflammatory effect of the -3 EFA which was given concurrently with Diclofenac Sodium. The results were in support of the studies which had stated that n-3 EFA had an anti-inflammatory activity [3–6, 16, 17].
The mean Diclofenac Sodium dose which was consumed per day concurrently with n-3 EFA was also analyzed. There was a significant difference in the mean Diclofenac Sodium dose which was consumed per day in each patient at different weeks of the treatment protocol. However, a significant reduction (p<0.05) in the mean dose (98.83 ± 22.31) of Diclofenac Sodium was recorded at 24 weeks as compared to the mean dose (115.04 ± 24.56) which was consumed at week 4 [Table/Fig-3].
Again, this decrease in the mean dose of Diclofenac Sodium could be due to the anti-inflammatory effect of the n-3 EFA which was used concomitantly with Diclofenac Sodium. Moreover, the reduction in the mean daily dose of Diclofenac Sodium could possibly decrease the side effects which were produced by it as an NSAID agent.
The percentage of the patients who had a ‘Better’ Subjective Response to the pain increased from 15.38% at week 4 to 61.54% at the end of the study [Table/Fig-4].
The results of this study revealed that the concomitant use of n-3 EFA with Diclofenac Sodium consumed reduced the dose of Diclofenac Sodium and probably the risk of side effects which were associated with it. n-3 EFA could possibly be used as an alternative to the NSAIDs in RA patients, to reduce the inflammation and in turn the risk of the side effects which were produced by the NSAIDs. The study reports the preliminary observations over a cohort of patients with Rheumatoid Arthritis.
Further studies which are conducted for a longer period with larger number of patients and with the use of an increased quantity of EPA and DHA in a single Cod liver oil capsule so that less number of capsules would have to be taken per day, thereby improving the patient compliance, may be required in the future, to explore the findings of the present study.