Nitric Oxide Levels in Patients with Chronic Renal Disease
S. R. Meenakshi1, Rajni Agarwal2
1 Assistant Professor, Department of Biochemistry, Malabar Medical College, Calicut, India.
2 Ex-Professor and HOD, Department of Biochemistry, M. S. Ramaiah Medical CollegeBangalore, India.
Name, Address, E-Mail Id of the Corresponding Author: Dr S R Meenakshi, Assistant Professor, Department of Biochemistry, 33/B, LIG II Stage, KHB Colony, Basaveswara Nagar, Bangalore–79, India.
Phone: 9741226190
E-mail: srmeenakshi@yahoo.com
Background and Objectives: Nitric Oxide (NO), the L-arginine derivative, is tonically synthesised by the endothelium within the kidney and it plays a crucial role in the regulation of the blood pressure and the renal blood flow. NO regulates the renal function through the modulation of the vascular tone and sodium handling. With the progressive development of the renal insufficiency, it remains unclear whether the endogenous NO production is increased or decreased in the kidney. This study was carried out to determine whether there were any changes in the levels of NO and teir correlation with the routine parameters of the renal dysfunction in the patients of Chronic Renal Failure (CRF), as the disease progresses in conjunction with poor renal functions.
Methods: Thirty patients with chronic renal disease which was caused by chronic glomerulonephritis and hypertension, who were on Maintenance Haemodialysis (MHD) with serum creatinine levels of > 2.5 mg/dl, were included in this study. Thirty healthy voluntary blood donors were taken as the controls. NO was estimated by a spectrophotometric method by using cadmium reduction.
The routine renal function tests, BUN and Creatinine were performed by the standard clinical chemistry procedures.
Results: The serum NO levels were found to be significantly increased (p < 0.01) in the CRF on MHD (98.77 ± 35.40 μmol/l) as compared to the controls (22.03 ± 7.23 μmol/l). The NO output correlated with the serum creatinine (r = 0.8123, p < 0.01) and the urea concentration (r = 0.5166, p = <0.01) in the CRF group.
Conclusion: The NO levels were markedly enhanced in the CRF patients who were on MHD. This was due to the dialysis procedure itself, which led to the stimulation of cytokine induced NO synthase and also due to the platelets which generated more NO due to uraemia. At high concentrations, NO is a cytotoxic molecule which is responsible for the complications of dialysis and it results in Nitrosative Stress in these patients, as it is a highly reactive free radical. Since the no output correlated with the serum creatinine and urea concentrations, a higher no production probably indicated insufficient blood purification, due to the common effect on their elimination pathways via the renal tract. Therefore, the alterations of the renal function, that are reflected in the changes of the creatinine concentration, will be accompanied by the changes in the serum NO. Thus, the determination of the NO levels in the peripheral blood may be useful in the assessment of the dialysis and they can also be used as markers in the follow up and the prognosis in these type of patients.
Chronic renal failure, Maintenance haemodialysis, Nitric oxide, Creatinine, Nitric oxide synthases
Introduction
Chronic Renal Failure (CRF) is a common renal problem, where the renal injury is of a more sustained nature, which is often not reversible, leading to a progressive destruction of the nephrons and culminating in glomerular and tubular insufficiencies [1]. In the past few years, Free radicals and Reactive Oxygen Species (ROS) were found to be the other important factors in the pathogenesis of chronic renal disease [2]. Nitric Oxide (NO) has recently been appreciated as an important ROS. NO has proven beyond doubt, to be a significant biological entity, because it had never been shown that a gas molecule could function as a signalling molecule in the body [3]. The inadequate NO production within the kidneys plays a key role in causing/or mediating the complex haemodynamic disorders which are associated with the progression of chronic renal disease. In CRF, there is extensive reduction of the Renal Mass (RMR), which is associated with the development of hypertension and it exhibits a decrease in both the GFR and the renal blood flow [4]. It has also been suggested that in renal failure, an NO deficiency could result from a reduced arginine availability, since the kidney is a major site of an endogenous arginine synthesis [5, 6]. However, a recent study which was done by Boudy et al., showed that the renal arginine synthesis and the arginine plasma levels were not diminished in the remnant kidneys, despite a significant reduction in the GFR [7]. Based on this, it is unlikely that the substrate supply of arginine would be rate limiting for the Nitric Oxide Synthases (NOS), even with the severe reduction of the functional renal mass, unless there are extraordinary arginine demands (i.e., sepsis or vigorous cytokine-induced NOS activation, as in dialysis). While NO serves a beneficial role as a messenger and a host defense molecule, excessive NO production can be cytotoxic, which is the result of NO’s reaction with the reactive oxygen and nitrogen species, leading to peroxynitrite anion formation, protein tyrosine nitration, and hydroxyl radical production [8]. Moreover, an impaired NO production has been implicated in the pathogenesis of volume—dependent hypertension. This duality of NO’s beneficial and detrimental effects has created extraordinary interest in this molecule and the need for a detailed understanding of the NO biosynthesis [8]. An impaired NO synthetic pathway could play a key role in modulating the complex renal haemodynamic disorders which are associated with the progression of renal diseases [9]. There is evidence which has indicated that a renal NO deficiency occurs in the patients with CRF. Thus, the daily excretion of urinary nitrate/nitrate was significantly lower in the patients with moderate and severe renal failure, as compared to that in those with mild renal failure and in the controls; the lowest values were found in the severe renal failure group [10]. This study would therefore be useful in determining whether there were any changes in the levels of serum NO in the patients with chronic renal disease, who were on Maintenance Haemodialysis (MHD), as the disease progresses in conjunction with poor renal functions.
Material and Methods
This study was carried out at M S Ramaiah Medical Teaching Hospital, Bangalore, India, in 2005, by including 30 patients of either sex, who were within the age group of 25–70 years as the cases. The patients who were clinically diagnosed as having chronic renal disease due to chronic glomerulonephritis and hypertension and who were on Maintenance Haemodialysis with serum creatinine levels of more than 2.5 mg dL=1 were included in the study. The samples were collected predialytically. The patients with chronic renal disease which was caused by diabetes mellitus, liver disease, lupus glomerulonephritis and other autoimmune disorders were excluded. The clinical history and the other necessary details were obtained from their case sheets. The study was conducted after informed consents were obtained from the patients and after the study was approved by the ethical committee of the institution. The control group consisted of 30 healthy males and females who were within the age group of 25–70 years.
Under aseptic precautions, 5 ml of fasting venous blood samples were collected. The blood was clotted and it was subjected to centrifugation. The clear serum was separated and it was used for the following biochemical investigations: Serum Nitric Oxide, Blood Urea Nitrogen (BUN) and Serum Creatinine. All the chemicals which were used were of the highest analytical grade, which are available in India.
Serum Nitric Oxide was determined by the greiss reaction as in Cortas and Wakid’s method [11]. NO is a labile and a diffusible molecule which forms stable metabolites (nitrite/nitrate) and these are detected by the greiss reaction. In the kinetic method, nitrate is reduced to nitrite by copper coated cadmium granules. This nitrite which is produced is determined by the diazotisation of sulfanilamide and coupling to Napthylethylenediamine to form a purple complex, which was measured at 545 nm by using a spectrophotometer. Serum creatinine and BUN were estimated by the standard clinical chemistry methods [12].
Results and Discussion
Statistically significant increases in the serum NO levels were found in the CRF patients who were on MHD, as compared to those in the controls (p < 0.01). Serum NO showed a significant positive correlation with serum creatinine (r = 0.8123) (p < 0.01) and BUN (r = 0.5166) (p < 0.01) in the CRF patients [Tables/Fig-1 and 2]. The correlation between serum NO and serum creatinine was more significant as compared to that between serum nitric oxide and serum urea nitrogen. We propose that this effect most probably resulted from a common effect on their elimination via the renal tract. Thus, the alterations of the renal function which are reflected by the changes in the creatinine concentration, will be accompanied by changes in the serum nitric oxide. There was a quadratic relationship between serum nitric oxide and serum creatinine, which indicated that serum nitric oxide had increased steeply after a certain value of creatinine (8 mg/dl) [Table/Fig- 3]. This could be attributed to the declining renal function and the insufficient purification of the blood. From this study, it has been found that the CRF patients who were on MHD had high levels of serum nitric oxide. The steady state levels of serum NO can be looked upon as a balance of the processes, namely the rate of entry into the circulation and the rate of elimination from the circulation or both. Normally, NO is eliminated by the process of glomerular filteration. In the subjects of the present study, NO could also be eliminated by the process of dialysis [13].
Comparison of parameters in controls and CRF on MHD patients
| Parameters | Controls (n = 30) Mean ± SD | Study Group (n = 30) Mean ± SD | Comparison |
|---|
| Serum NO (μ mol / L) | 22.03 ± 7.23 | 98.77 ± 35.40 | p<0.01 highly significant |
| Serum Creatinine (mg dL-1) | 0.95 ± 0.16 | 9.49 ± 2.93 | p<0.01 highly significant |
| BUN (mg dL-1) | 10.47 ± 2.61 | 70.83 ± 19.43 | p<0.01 highly significant |
Correlation between Serum NO, Serum creatinine and BUN
| Correlation between parameters | CRF patients | r-value | p-value | Level of significance |
|---|
| Serum NO and Creatinine | Serum NO | Serum Creatinine | 0.8123 | <0.01 | Highly significant |
| 98.77±35.40 | 9.49±2.93 | | | |
| Serum NO and BUN | Serum NO | Serum BUN | 0.5166 | <0.01 | Highly significant |
| 98.77±35.40 | 70.83±19.43 | | | |
Correlation of serum Nitric oxide and serum creatinine in the study group
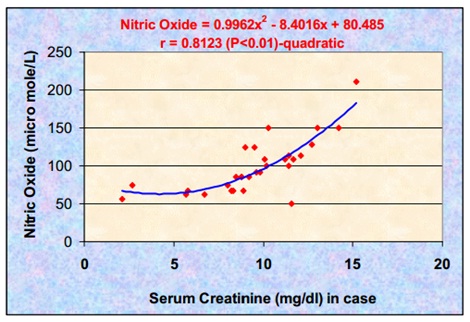
Since serum NO itself is known to be eliminated by the kidneys, largely by the process of glomerular filteration at the rate of 20 ml/min, elevated serum NO could therefore be taken as a representative of the declining renal function.
It is well known that MHD, as a therapeutic option, has several limitations. The serum creatinine and the urea levels often fall marginally after these sessions. In the subjects of CRF, who were on MHD, the mean serum creatinine and the serum urea continued to remain respectively high.
Similarly, there was no appreciable fall in the serum NO, despite the subjects being on regular sessions of dialysis. Nitric oxide and its metabolites are small diffusible molecules which should normally be easily eliminated by dialysis. It is therefore possible that there are other factors which contribute to the increased NO levels, which may be an increased endogenous production, the hyperactive L-arginine/Nitric oxide synthetic pathway and the activation of the immune system by the dialysis procedure itself, leading to the induction of iNOS [14] and also the platelets which generate more NO due to uraemia [15]. At high concentrations, NO is a cytotoxic molecule which is responsible for the complications of dialysis and it results in Nitrosative Stress in these patients, as it is a highly reactive free radical. The extension of this study may also provide an insight into a better understanding on the possible role of NO in the complications of dialysis like hypotension [16] and in assessing the extent of the Nitrosative Stress in the CRF patients who are on dialysis [17].
Research into the regulation of the NO synthases activity and the development of NOS inhibitors for blocking the specific isoforms of NO, as well as the stable compounds that release it, would be a major challenge for the therapeutic development [18]. The comparison of the two proposed vital parameters, i.e., serum NO and serum creatinine, may find its use as an indicator of the prognostic follow up in the chronic renal failure patients who are on dialysis. A humble beginning has been made, which if followed, would prove useful for the CRF patients who are on dialysis. Further avenues for generating novel ideas and designing studies to address the queries which are related to its real significance, have to be carried out.
[1]. Klahr S, Schriener Ichikawa I, The progression of renal diseaseN Eng J Med 1988 25:1657-66. [Google Scholar]
[2]. Davis ConferenceOxygen Radicals and human diseaseAnn Int Med 1987 107:526-45. [Google Scholar]
[3]. Stratta Piero, Canvese Caterina, Dogliani Margherita, The role of free radicals in the progression of renal diseaseAm J Kid Dis 1991 17:33-37. [Google Scholar]
[4]. Aiello S, Noris M, Todesehini M, Renal and systemic NO synthesis in rats with renal mass reductionKidney Int 1997 52:171-81. [Google Scholar]
[5]. Mitch W, Chesney R, Amino acid metabolism by the kidney. MinerElectrolyte Metab 1983 9:190-202. [Google Scholar]
[6]. Chan W, Wang M, Kopple J, Swenseid M, Citrulline levels and urea cycle enzymes in uremic ratsJ Nutr 1974 104:678-83. [Google Scholar]
[7]. Boudy N, Hassler C, Parvy P, Bankir L, Renal synthesis of arginine in CRF; In vivo and in vitro studies in rats with 5/6 nephrectomyKidney Int 1993 44:676-83. [Google Scholar]
[8]. Kone Bruce C, Nitric oxide in renal health and diseaseAm J Kid Dis 1997 30:311-33. [Google Scholar]
[9]. Matsumoto Akihiro, Hirata Yasunobu, Increased excretion of Nitric oxide in exhaled air of patients with chronic renal failureClin Science 1999 96:67-74. [Google Scholar]
[10]. William N, NO news is good news but only for three AmericansScience 1998 282:610-11. [Google Scholar]
[11]. Najwa Cortas, Nabil Wakid, Clin Chem 1990 36:1440-42. [Google Scholar]
[12]. Talke H, Schubert GE, “Enzymatische Harnstoffbest immung in Blut und Serum in optischen est nach Warburg”Klin wschr 1965 41:174 [Google Scholar]
[13]. Fayed HM, Kamal MA, Sultan MM, Mowafy MN, Nitric oxide generation by the peripheral blood cells in CRFBr J Biomedical Sci 2002 :34-39. [Google Scholar]
[14]. Sarkar Shubho R, Charoen Kaitwatcharachai., and Nathan W Levin.: Basic science and dialysis: Nitric oxide and hemodialysisSeminars in dialysis 2004 17(3):224-28. [Google Scholar]
[15]. Tatiana M, Brunini C, Antonio C, Mendes-Ribeiro Ellory John C, Platelet nitric oxide synthesis in uremia and malnutrition: A role for L-Arginine supplementation in vascular protection?Cardiovasc. Res 2007 73(2):359-67. [Google Scholar]
[16]. Nambu Akira, Hara Katsuko, Enhanced production of nitric oxide may be involved in acute hypotension during maintenance hemodialysisAm J Kidney Dis 1998 31:809-17. [Google Scholar]
[17]. Nath KA, Goalt AJ, Hostetter TH, Effect of dietary protein restricton on the O2 consumption and oxidant stress on the remnant nephronKidney Int 1988 33:381 [Google Scholar]
[18]. Viteak Jan, Lojik Antonin, Valachi Guiseppe, Arginine base inhibition of Nitric oxide Synthase; Therapeuic potential and challengesMediators of Inflammation 2012 :22pages [Google Scholar]