Myoepithelial Carcinoma of the Breast
Santhosh R1, Kemba Padu2, Bipin Singh TH3, M Birkumar Sharma4, TH. Sudhir Chandra Singh5
1 Junior Resident, Department of Surgery, Regional Institute of Medical Sciences, Imphal, India.
2 Junior Resident, Department of Surgery, Regional Institute of Medical Sciences, Imphal, India.
3 Senior Consultant, Department of Pathologist, Babina Diagnostics, Imphal, India.
4 Professor, Department of Surgery, Regional Institute of Medical Sciences, Imphal, India
5 Professor, Department of Surgery, Regional Institute of Medical Sciences, Imphal, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. M Birkumar Sharma, Professor, Department of Surgery, Regional Institute of Medical Sciences, Imphal, India.
Phone: 9774867590
E-mail: drsanthoshr@ymail.com
Breast carcinoma is the commonest cancer among females in a majority of the Indian cities. The advances in the research and management have improved the breast cancer survival significantly in the past three decades globally. Adenocarcinoma is the commonest histological variant which arises from the ductal epithelia. The myoepithelial cells (ME) are the normal components of the breast parenchyma, which separate the ductal epithelia from the basement membrane and the stroma. The pure ME cell carcinoma is extremely rare and only 38 cases have been reported so far in the indexed literature. This may owe to the difficulties in the identification, and the non availability of established diagnostic criteria. We herein describe the clinical, radiological and the pathological characteristics of a case of myoepithelial carcinoma, to supplement the available literature. The possible impact of these cells in clinical practice was also reviewed. Identification and the further research on the genesis of these tumours, and the pathways by which the ME cells regulate the milieu interior of the breast epithelia, may unravel new molecular targets to prevent or treat both epithelial and myoepithelial cancers at early stages.
Myoepithelial carcinoma, Carcinoma breast
CASE REPORT
A 73-year-old postmenopausal, compensated hypertensive lady who was on anti-hypertensives presented with a palpable and a non tender, lump in the left breast, which she noticed three months ago. It had progressively increased in size since then. She had attained menarche at 13 years of age, and had got married at 20 years. She had four children. They were all breast fed for at least 18 months. She attained menopause 20 years back. There was no history of breast or any related cancer in her family. The examination of her left breast showed a well defined solitary swelling, which measured 8x7 cm in the lower outer quadrant of the left breast. It was non-tender and firm, with an irregular surface and well defined margins. There was no involvement of the skin, nipple, the areolar complex or the pectorals. The opposite breast was normal. Bilateral axillae had no palpable lymph nodes. The rest of the examination was unremarkable. The clinical diagnosis was of carcinoma of the left breast in the lower outer quadrant, which was a T3N0M0 tumour according to the AJCC (American Joint committee on Cancer), 7th edition.
The fine needle aspiration cytology smears which were studied were moderately cellular, with the cells being arranged in clusters and scattered as singles in a myxoid background. The individual cells were pleomorphic, with variable amounts of cytoplasm, and pleomorphic vesicular nuclei with distinct nucleoli. The features were of an infiltrating ductal carcinoma. Ultrasonography of the tumour showed a fairly well defined, oblong, lobulated solid mass lesion in the lower outer quadrant of the left breast, which measured 8.1×6.1×7.7 cm (CC×AP×AL), which was approximately at 1.5 cm from the nipple margin. Increased vascularity was seen on Doppler analysis, with low resistance waveforms. The elastography examination showed a predominantly hard consistency within the lesion, in both the soft and hard compressions. There was no evidence of axillary lymphadenopathy. The features were of a BIRADS 4 lesion on ultrasonography and mammography. The other breast was radiologically normal. The workup for a distant metastasis was negative, with normal liver function tests, X-rays of the chest and spine, and ultrasound of the abdomen.
Under general anaaesthesia, the patient underwent a simple mastectomy with levels 1 and 2 axillary lymph node clearance (Auchincloss`s modified radical mastectomy). The gross specimen measured 19×12×9 cm and it was covered by an elliptical bit of skin which measured 15×10 cm. The serial cut sections showed a tumour which measured 6×5×5 cm in maximum dimension. The cut surface was firm and grey white and the margins were relatively well preserved. The rest of the breast tissue appeared unremarkable on the macroscopic examination. Eighteen lymph nodes were isolated, the largest measuring 0.7×0.5 cm.
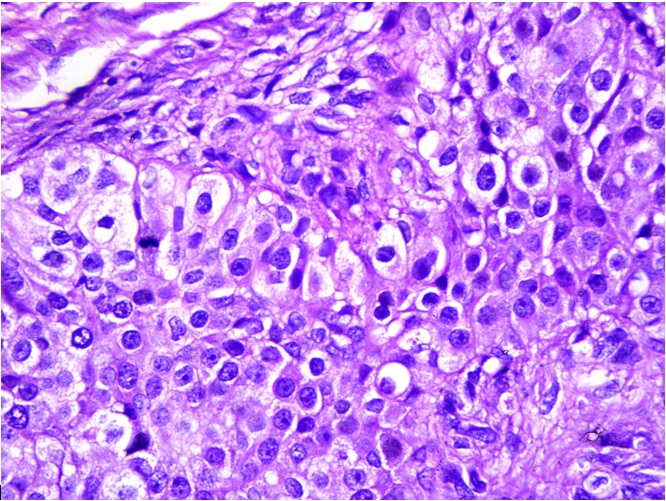
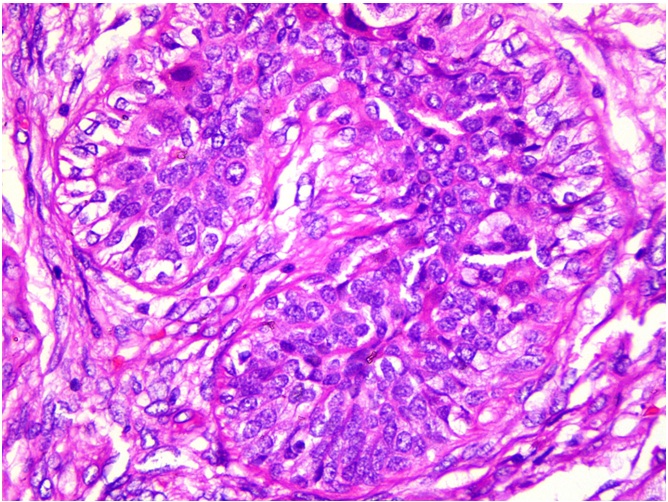
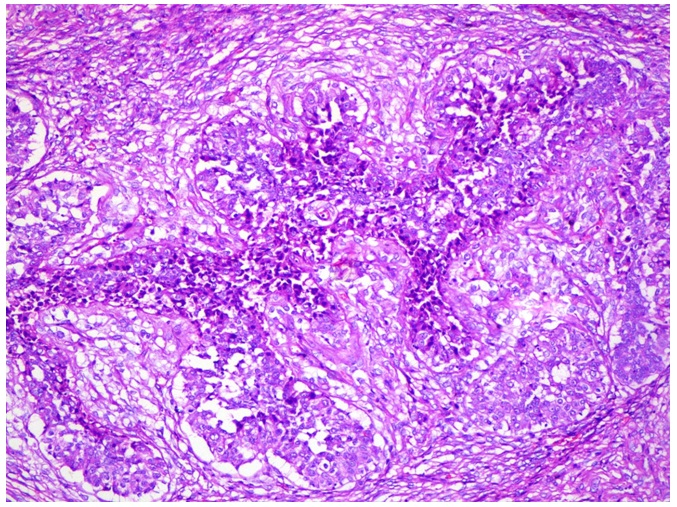
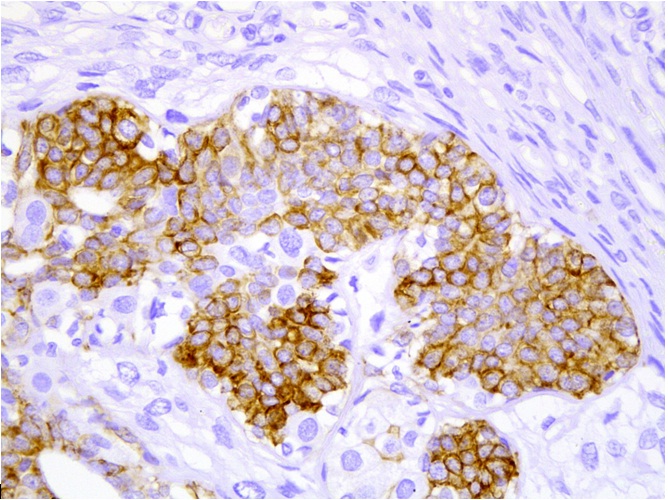
Multiple sections were studied and they showed breast tissue with a malignant tumour which was composed of epitheloid to polygonal cells which had moderate amounts of cytoplasm and pleomorphic vesicular nuclei with distinct nucleoli, which were arranged predominantly in lobules and occasional fascicles [Table/Fig-1]. Many foci displayed cells with clear cell changes [Table/Fig-2]. Numerous abnormal mitoses were seen. Also seen were foci which displayed a biphasic pattern with mild to moderate nuclear atypia. Focal areas with the features of adenomyoepithelial adenosis were noted. The tumour was found to diffusely infiltrate the stroma [Table/Fig-3]. There was an evidence of focal areas of ductal carcinoma in situ. No lymphovascular emboli or an evidence of a perineural spread was seen. All the surgically resected margins (1.5 to 7 cm) were negative for malignant cells. Immunohistochemistry was performed and the tumour cells were seen to be positive for SMA, CD 10 and S-100 (4+) [Table/Fig-4], and negative for ER, PR and Her-2/neu. The dysplastic lining epithelial cells were positive for pan CK, ER, PR and c-erbB-2/Her-2/neu. The histopathological features were of a myoepithelial carcinoma with a ductal carcinoma in-situ (flat type), which had risen in a background of adenomyoepithelial adenosis. The patient had an uneventful postoperative stay. She received 6 cycles of an anthracycline based chemotherapy with whole breast irradiation with Co-60, and is being followed up with the hormonal therapy, anastrozole 1mg once daily.
Tumor composed of epitheloid to polygonal cells having pleomorphic vesicular nuclei with distinct nucleoli
Tumor with foci displaying cells with clear cell change
Section showing the diffuse infiltration of stroma by malignant cells
Immunohistochemistry showing malignant cells staining positive for S-100
DISCUSSION
The normal breast tissue consists of branching ductal networks which are lined by an outer layer of myoepithelial cells and an inner layer of polarized luminal epithelium. They regulate the cell cycle, growth and differentiation of the ductal epithelium, from which the commoner adenocarcinomas arise [1]. The ME cell layer forms a continuous envelope over the epithelium in the ducts whereas it only forms a scaffold in the lobules, leaving some epithelial cells in direct contact with the basement membrane which separates the stroma [2]. This tumour microenvironment and the differential expressions of the ME cells may also explain the different clinical behaviours of the ductal and the lobular neoplasia. Both cells are derived from the common precursor cells which are cytokeratin (CK) 5 positive. The ME cells stain variably with CK 5 and CK 14/17, in contrast to the CK 5 and the CK8/18/19 positivity of the luminal epithelia [3]. ME cells can transform to form neoplasia of varying grades. A myoepithelial carcinoma [MEC], in its pure form, is an extremely rare tumour [4].
The ME cells express tumour suppressors which include angiogenesis and proteinase inhibitors like maspin, p63 and WT-1 [5]. Through paracrine signalling they maintain the epithelial cell polarity and inhibit the cell migration and invasion [2]. The presence of ME cells is considered a sign of benignity of a breast lesion, the numbers of which decrease with the advancing grades of neoplasia [6]. Increasing research has identified ME cell specific genes like S100A2, LGALS7, CSTA and BPAG [1]. The expression of SPARC [osteonectin] is known to independently portray a poor prognosis in breast carcinoma, which is irrespective of the ER/PR status. These markers can be used in the identification of the myoepithelial cells as well [7].
A fully differentiated myoepithelial cell acquires a contractile phenotype due to the cytoplasmic α- smooth muscle actin (SMA) and the heavy chain myosin. Depending on the degrees of differentiation, they variably show staining for SMA, vimentin, calponin, S-100 (Epithelial membrane antigen), NGFR, CD 10, and EGFR [8]. The myoepithelial cells are mitotically quiescent with a low proliferative index, but they can be transformed [9]. The spectrum of the neoplasms does include adenomyoepithelioma, malignant adenomyoepithelioma with the degeneration of either or both the components and a pure myoepithelial carcinoma which is extremely rare. Though they are believed to be low grade malignancies, an aggressive clinical behaviour is documented in more than 50% of the cases with predominant haematogenous metatstases with a propensity for a local recurrence [4,10]. The paradox remains unexplained. They are fast growing tumours, as was also seen in our case. Though tenderness has been reported, it was absent in our case [11].
The diagnosis on aspiration cytology may be successful as described by Sauer if spindle cells are seen [12]. An intraductal MEC diagnosed by cytology is also described. But the sampling of the non-representative areas or the presence of a myxoid background makes the diagnosis of a myoepithelial carcinoma less straight forward though should suggest. Thus, a small core needle biopsy would be more appropriate [13].
The diagnostic confusion continues even after the identification of the spindle cells as a metaplastic (sarcomatoid) carcinoma, spindle carcinoma, malignant fibrous histiocytoma and other sarcomas may demonstrate the common stigmata of cancer [14]. The presence of a myxoid stroma was believed to point towards other lines of differentiation, but recently, in the salivary glands, the morphologic spectrum of the myoepithelial cells has been expanded to include the tumours with a myxoid or a hyalinized stroma, a reticular or a trabecular architecture and epitheloid or clear myoepithelial cells [15]. A similar extension can be considered for the ME cells of the breast. Recently, a rhabdoid differentiation was also reported [4]. A lack of familiarity with the range of the appearances, may at least in part be responsible for the paucity of the recognized cases [6]. Extending the application of the myoepithelial markers to small needle core biopsies rather than merely relying on the myoepithelial layer for differentiating the DCIS from an invasive carcinoma, can make significant contributions to the clinical practice by reducing false negatives. In our case, the positivity for pancytokeratin, SMA, S 100 and CD 10 clearly proved the myoepithelial origin of the present lesion. The aggressive clinical behaviour which has been reported in the literature warrants an aggressive local treatment and a systemic adjuvant therapy.
CONCLUSION
Myoepithelial carcinoma of the breast is rare and difficult to diagnose owing to its varied morphological characteristics. Its clinical, radiological and histopathological characteristics are described herein. There is a need for the establishment of standard criteria to aid in its identification and correct categorization. In view of the paucity of management guidelines and the possible propensity for a local recurrence and the metastatic behaviour which have been reported, an aggressive local treatment with an adjuvant chemo-radiotherapy should be the standard of care even in the small tumours. Identification and further research on the genesis of these tumours, and exploring the pathways by which the ME cells regulate the milieu interior of the breast epithelia may unravel new molecular targets for preventing or treating both the epithelial and the myoepithelial cancers at early stages. All such tumours merit reporting henceforth, to throw more light on them.
[1]. Liao KC, Lee WY, Chen MJ, Myoepithelial Carcinoma: A Rare Neoplasm of the BreastBreast Care [Basel] 2010 Aug 5(4):246-49. [Google Scholar]
[2]. Polyak K, Hu M, Do myoepithelial cells hold the key for breast tumor progression?J Mammary Gland Biol Neoplasia 2005 Jul 10(3):231-47. [Google Scholar]
[3]. Böcker W, Moll R, Poremba C, Holland R, Van Diest PJ, Dervan P, Bürger H, Wai D, Ina Diallo R, Brandt B, Herbst H, Schmidt A, Lerch MM, Buchwallow IB, Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: a new cell biological conceptLab Invest 2002 Jun 82(6):737-46. [Google Scholar]
[4]. Ohtake H, Iwaba A, Kato T, Ohe R, Maeda K, Matsuda M, Myoepithelial carcinoma of the breast with focal rhabdoid featuresBreast J 2013 Jan-Feb 19(1):100-3.doi: 10.1111/tbj.12058. Epub 2012 Dec 17 [Google Scholar]
[5]. Barsky SH, Karlin NJ, Myoepithelial cells: autocrine and paracrine suppressors of breast cancer progressionJ Mammary Gland Biol Neoplasia 2005 Jul 10(3):249-60. [Google Scholar]
[6]. Ahmed AA, Heller DS, Malignant adenomyoepithelioma of the breast with malignant proliferation of epithelial and myoepithelial elements: a case report and review of the literatureArch Pathol Lab Med 2000 Apr 124(4):632-6. [Google Scholar]
[7]. Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancerCancer Res 2004 May 1 64(9):3037-45. [Google Scholar]
[8]. Kaya H, Güllüoglu B, Aribal E, Myoepithelial differentiation in breast carcinomaTumori 2008 Jan-Feb 94(1):116-20. [Google Scholar]
[9]. Joshi K, Smith JA, Perusinghe N, Monoghan P, Cell proliferation in human mammary epithelium. Differential contribution by epithelial and myoepithelial cellsAm J Pathol 1986 Aug 124(2):199-206. [Google Scholar]
[10]. Lakhani SR, O’Hare MJ, The mammary myoepithelial cell--Cinderella or ugly sister?Breast Cancer Res 2001 3(1):1-4. [Google Scholar]
[11]. Tavassoli FA, Myoepithelial lesion of breast. Myoepitheliosis, adenomyoepithelioma and myoepithelial carcinomaAm J Surg Pathol 1991 Jun 15(6):554-68. [Google Scholar]
[12]. Sauer T, Cytological findings in malignant myoepithelioma: a case report and review of literatureCytojournal 2007 Jan 25 4:3 [Google Scholar]
[13]. Woo EK, James AD, Mercer J, Allan SM, Howlett DC, Case report: myoepithelial carcinoma of the breast: a case report with imaging and pathological findingsBr J Radiol 2005 May 78(929):444-6. [Google Scholar]
[14]. AI-Nafussi A, Spindle cell tumours of the breast: practical approach to diagnosisHistopathology 1999 Jul 35(1):1-13. [Google Scholar]
[15]. Hornick JL, Fletcher CD, Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemistry study of 101 cases with evaluation of prognostic parametersAm J Surg Pathol 2003 Sep 27(9):1183-96. [Google Scholar]