Photodynamic Therapy: The Imminent Milieu For Treating Oral Lesions
Neeta Mohanty1, MD Jalaluddin2, Sreekanth Kotina3, Samapika Routray4, Yashwant Ingale5
1 HOD & Professor, Department of Oral Pathology & Microbiology, Institute of Dental Sciences, Bhubaneswar, Odisha, India.
2 Reader, Department of Periodontics & Oral Implantology Kalinga Institute of Dental Sciences, Khushabhadra, Campus-5, KIIT University, Patia, Bhubaneswar, Odisha-750024, India.
3 Senior Lecturer, Department of Oral Pathology & Microbiology, GITAM Dental College & Hospital, Gandhinagar Campus, Rushikonda, Vishakapatanam, Andhra Pradesh-530045, India.
4 Senior Lecturer, Department of Oral Pathology & Microbiology, GITAM Dental College & Hospital, Gandhinagar Campus, Rushikonda, Vishakapatanam, Andhra Pradesh-530045, India.
5 HOD, Department of Dentistry, Y.C.M Hospital, Pimpri, Pune, Maharastra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORESPONDING AUTHOR: Dr. Samapika Routray, Department of Oral Pathology & Microbiology, GITAM Dental College & Hospital, Gandhinagar Campus, Rushikonda, Vishakapatanam, Andhra Pradesh-530045, India.
E-mail: drroutray.samapika@gmail.com
Photodynamic therapy (PDT) is used in curative and palliative treatment of head and neck squamous cell carcinoma (HNSCC) and other oral lesions. Oral infections (such as mucosal and endodontic infections, periodontal diseases, caries, and peri-implantitis) are among the specific targets where PDT can be applied Photodynamic therapy (PDT) efficacy depends on the local dose deposited in the lesion as well as oxygen availability in the lesion. Further long-term clinical studies are necessary in establishing a more specific place of the technique in the field of dentistry.
INTRODUCTION
Photodynamic therapy (PDT) is a relatively new method for treating superficial tumours of the skin and the mucosa. Prof. Hermann von Tappeiner was the person to coin the term “photodynamic”, to describe oxygen-consuming chemical reactions in vivo [1]. Currently, photodynamic therapy (PDT) is a treatment modality which involves the administration of a photosensitizing compound and the accumulation of sensitizer molecules in the target cells, followed by a selective irradiation of the lesion with visible light. Drug and light are individually non-toxic and in combination, they destroy tissues. In recent times, Canada, France, Germany, Japan, The Netherlands and U.S. have approved PDT for treating select malignancies which are intraoperative and for intracavitary use. It is also used as an investigational treatment in diseases likes psoriasis vulgaris, warts, diseases of the epidermal appendages, atherosclerosis, rheumatoid arthritis and bacterial infections.
EVOLUTION OF PDT
Oscar Raab [2], a German medical student, first observed the death of Paramecium caudatum after a light exposure in the presence of acridine orange. Then von Tappeiner and Jesionek (a dermatologist), in 1904 [3], used topical eosin and visible light to treat skin tumours, condyloma lata and lupus vulgaris. Later, Policard, Auler and Banzer, Figge, Weiland and Manganiello, independent of each other, reported the tumour localization of haematoporphyrin. Dougherty et al., pioneered the successful use of PDT to treat cutaneous cancers and other malignancies [4].
PHOTODYNAMIC THERAPY MECHANISMS
Photodynamic therapy utilizes the combination of light and a photosensitizer to bring about a cytotoxic effect to cancerous or otherwise unwanted tissues. It employs the local activation of a photosensitizer which gets accumulated in a tumour, by means of the exposure to the light of an appropriate wave length. The direct cytotoxic effect of the photochemical reaction is due to the induction of necrosis and apoptosis by singlet oxygen species. It can however, bring about indirect damage to the tumour cells by causing vascular endothelium damage by initiating an immune response against the remaining tumour cells [Table/Fig-1] [5].
Photodynamic therapy mechanism.
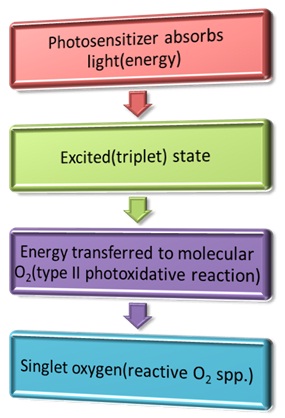
The PDT induced effects are mediated by the photo-oxidative reactions, type I and II, of which type II is more important. In type, peroxide, superoxide and hydroxylions are used. The major biologic effects of this process are the cellular part and secondarily, the vascular (systemic PDT) effect. The other effects which are associated are inflammatory mediators access and immune stimulation. There is no DNA damage primarily and so there is a negligible risk of mutations or carcinogenesis [6].
The cumulative effects inside the cells are described as:
Lipid peroxidation of the cell membrane, leading to cell lysis
Damage to the mitochondria, lysosomes and the endoplasmic reticulum
Apoptotic biochemical markers appear
Vasoconstriction, blood stasis and tumour vessel thrombosis. leading to ischaemic necrosis
Phospholipid degeneration and vascular phenomena, leading to an inflammatory mediator release, which mediates granulocyte and macrophage aggregation.
Generation of tumour specific immune cells
THE TYPES AND MODALITIES OF PDT
The variables in PDT are:
Photosensitizers: This may be systemic or topical
Oxygenation of the tissues: Experimental attempts have been made at improving the effectiveness of PDT by manipulating the tumour O2 content, but it has not been proven clinically as yet.
Light source: Lasers (coherent) and incoherent sources
Photodynamic therapy systemic photosensitizers [7]
The haematoporphyrin derivative (HpD) and the porfimer sodium (Photofrin II) mixtures of non-metallic oligomeric porphyrins are used. Many normal tissues, especially RES, have a greater affinity for these than tumour tissues. These are usually activated by using light at 630nm. Though there is a greater light absorption by light at lower wavelengths, the tissue penetration by light is less.
The systemic photosensitizers which are still under research are tin ethyl etiopurpurin, Lutetium texaphyrin, BPD-MA (690, 3-5), M-THPC (652), and NPe6 (660). The characteristics which are desired are tumour specificity and strong at 650nm and above.
MAB-sensitizer conjugates
Photodynamic therapy topical photosensitizers [8]
5-aminolevulinic acid/ protoporphyrin IX (PpIX): The 1st haem precursor after the feedback control point. The topical ALA application subverts the negative feedback control of the haem synthesis pathway. This is a significant step but there is a short-lived (24 hr) intracellular concentration of PpIX. It can be activated with 630 nm light and modified by using desferrioxamine, DMSO, IL, etc.
The other topical photosensitizers which are under research are ATMPn and m-THPC
Photodynamic therapy ideal photosensitizer
It should be chemically pure and it should have a high singlet oxygen quantum yield. It requires significant light absorption at wavelengths that penetrate the skin sufficiently deeply and high tissue selectivity. Its efficacy increases after a topical application [9].
Photodynamic therapy light sources [10]
The therapeutic window should be about 600nm to 1200nm. The lasers that can be used are:
I Around 630nm is required to match the absorption maxima of the porphyrin photosens.
Argon pumped dye lasers (630) or gold vapour lasers (628) can be used, but these are expensive and they need regular maintenance.
Nd-YAG cover 690-1100 nm for the 2nd generation photosets.
Commercially available lamps with the appropriate red light emission for large surface areas-the metal halogen lamp (600-800nm high power density) and for small surface areas-the arc xenon lamp (400-1200nm). The radiance power at the periphery is less.
Photodynamic therapy light dosimetry [11]
The power density of light (energy fluence rate) is defined as the radiant energy which is incident per sec, divided by the cross sectional area of the irradiated spot which is given in W/m2.
The fluence rate X irradiation time=energy fluence (J/m2).
The light dose regimens in J/cm2 are empirical and they vary, depending on the location, size and the histopathologic type of the lesion.
Photofrin and 630nm light need fluences from 25-300 J/cm2; interstitial applications need radiant exposures between 100 and 400J/cm2; Topical Ala with laser 60-250 J/cm2 and 30-540 J/cm2 for incoherent light sources.
PDT POTENTIAL INDICATIVE DISEASES
Oncologic
Actinic keratosis
Bowen’s disease
Superficial Basal Cell Carcinoma/Squamous Cell Carcinoma
Nevoid Basal Cell Carcinoma syndrome
Keratoacanthoma
Kaposi’s sarcoma
Cutaneous metastases
Cutaneous T Cell Lymphoma, Xeroderma Pigmentosum and actinic cheilitis
Nononcologic
Psoriasis vulgaris
Human papiloma virus-associated dermatoses
Epidermodysplasia verruciformis
Condylomata acuminata
In actinic keratoses, the facial lesions respond better than the lesions which are at the trunk and the extremities. The thick hyperkeratotic lesions respond poorly.
PDT is useful for the widespread lesions of Bowen’s disease, especially for those which are in large patches and in anatomically difficult areas. It is better than liquid N2.
Mycosis fungoides: Encouraging results with topical ALA-PDT with Nd-YAG laser.
Topical and systemic applications of PDT have shown good results in BCC, except in nodular and morpheaform lesions. A moderate response was seen in pigmented BCC. It is valuable for multiple recurring BCC in Gorlin’s syndrome.
Topical PDT is effective in carcinoma in situ and in early invasive SCC, if the conventional therapy not practical. It is a useful diagnostic and an adjuvant therapeutic tool in advanced SCC.
In Kaposi’s sarcoma, the PDT response rate is enhanced along with other modalities.
PDT, in its current form, is not acceptable in Multiple myeloma.
Lip dysplasia which is caused by nicotine abuse is manageable with PDT
It is effective in actinic cheilitis, where cryotherapy, CO2 laser, 5FU and retinoids had failed earlier.
Good results are observed in keratoacanthomas.
It is inefficient in cutaneous liposarcomas
It is efficient in recalcitrant common warts
It is an efficient modality with excellent cosmesis in cutaneous vascular malformations
The low fluences promote hair growth and the high light doses lead to permanent hair loss without affecting the dermis which is adjacent to the hair follicles
PHOTODYNAMIC THERAPY - ADVERSE EFFECTS
Burning pain, stinging or itching which are restricted to the illuminated area during the light exposure. They rarely continue for few hours
Erythema and mild oedema of the treated area
A light overdose causes blistering, ulceration or excessive necrosis
A cutaneous photosensitivity with systemic PDT for 4 to 6 weeks, even upto 6 months
Residual hyperpigmentation and hypopigmentation, but they resolve soon
Allergic reactions like urticaria to the photosensitizers, are seen.
Systemic PDT with the use of various sensitizers can cause nausea, vomiting and liver function abnormalities
It is hazardous in porphyria. Especially, ALA penetrates the blood brain barrier, leading to neurologic symptoms.
It can aggravate systemic lupus erythematosus
Koebner reaction of psoriasis during the treatment of AKs and SCCs, which is induced by UVB for psoriasis.
PDT IN ORAL LESIONS
Photodynamic therapy is an important treatment option for the patients who present with recurrent carcinomas or secondary tumours of the upper aerodigestive tract and who have failed or have been unsuitable for other treatments. Oral erythroplakia (OEL) and oral verrucous hyperplasia (OVH) which are considered to have the highest malignant transformation potentials among all the precancerous lesions, showed better clinical outcomes with PDT in about 66–95% of the patients [12, 13]. But the treatment of oral leukoplakia (OL) still hasn’t shown high outcomes.
The tumours with smaller sizes (<1.5 cm) and lesser surface keratin layer thicknesses (630 lm) showed better PDT responses too. Due to the excellent treatment results that have been achieved so far [Table/Fig-2], it is anticipated that PDT may, in the future also, play a role in the primary treatment of superficial tumours of the oral cavity, the pharynx and the larynx. Efforts are being made for using PDT for other oral lesions, for example, oral lichen planus, candida adhered lesions, etc but they are in the preliminary stages. Its efficacy in oral leukoplakia is still be tested by using its various other modalities.
Studies showing positive outcome of PDT in oral lesions.
| Study | Cases | Treatment | Result |
|---|
| Grant et al., [14] | 11 patients with field cancerization | Photofrin 2mg/kg48 hours prior to photoirradiation with 50-100 J/cm2 red laser light by surface illumination | 10 of the 11 patients showed a complete response to PDT; 1 patient had areas of residual leukoplakia.2 patients developed further areas of leukoplakia or erythroplakia within 12 months but no patient has had evidence of recurrent invasive carcinoma in the treated areas. |
| Fan et al., [15] | 18 patients with histologically proven premalignant and malignant lesions | Photosensitizing agent 5 aminolevulinic acid (ALA) sensitized with 60 mg/kg ALA by mouth and treated with laser light at 628 nanometers (100 or 200 Joules/cm2). | 12 patients with dysplasia showed improvement.Out of remaining 6 patients with squamous cell carcinoma, only 2 became tumor free. |
| Kulapadi-tharom [16] | Fifty-one patients were treated over a period of 5 years. | | 91.67% complete response rate was observed for T1 tumors (primary and recurrence) with a recurrent rate of 27.27 per cent Nasopharyngeal carcinoma was highly responsive to PDT. PDT was remarkably effective in curing premalignant diseases (100% complete response rate). |
| Schweitzer; [17] | 10 patients with early stage Tis-T2N0M0 SqCellCarcinoma (SqCCa) of the oral cavity and oropharynx and 10 patients with Tis-T2N0M0 SqCCa of the larynx | Intravenous PHOTOFRIN (porfimer sodium) (dose 2.0 mg/kg) was administered ,followed 48-60 hours later by intraoperative light photoactivation at 630 nm via fiberoptic microlens (ML) delivery (surgical light dose, 50-100 J/cm(2)) and/or cylindrical diffuser (CD) delivery (80-100 J/cm). | Complete responses (CRs) (follow up 6 months-9 years) were achieved in eight of 10 patients with diffuse field cancerization of the oral cavity. CRs (follow up 6 months-8 years) were achieved in eight of 10 patients with superficial laryngeal cancer obviating the need for total laryngectomy in previously treated radiation therapy patients. |
| Lorenz et al., [18] | 35 patients with recurrent squamous cell carcinoma or secondary tumours of the head and neck | Meta-tetrahydroxyphenylchlorin (mTHPC), known under the trade name of Foscan, was used as the photosensitizing agent. | Local control was achieved in 21 patients (60%) and partial remission in 10 patients (28.5%). Four patients (11.5%) did not respond to PDT treatment. The mean duration of overall survival was 401.45 (+/-321.2) days, median was 356 after the completion of treatment. The mean duration of recurrence-free survival was 327.7 (+/-131.1) days, median was 181 for patients with complete remission. |
| Schweitzer; [19] | 30 patients with Tis-T2N0M0 SqCCA | Intravenous PHOTOFRIN (porfimer sodium) (dose 2.0 mg/kg) was administered outpatient, followed 48-60 hours later by intraoperative photoactivation at 630 nm via fiberoptic microlens surface delivery (light dose 50-100 J/cm(2)) or interstitial implantation via cylindrical diffuser fiberoptic delivery (light dose 50-100 J/cm). | Twenty-four of 30 patients (80%) have demonstrated complete remission (follow-up 3-144 months). Six patients who had partial remission with recurrence observed at 3, 3, 5, 9, 23, and 26 months subsequently retreated with conventional therapy. Eleven of 24 patients were cancer disease free at 2 years following PDT. |
| Hsu et al., [20] | 20 cases of 7,12-dimethylbenz(a)anthracene (DMBA)-induced hamster buccal pouch precancerous lesions were treated | Topical ALA-PDT with a light dose 75 J/cm(2) or 100 J/cm(2) using a 640-nm light-emitting diode (LED) light | 75-J and 100-J topical ALA-PDT treatment modalities are very effective for DMBA-induced hamster buccal pouch precancerous lesions. |
| Costa et al., [21] | 56 immunosuppressed mice with buccal candidiasis | Erythrosine (400 μmol/L) followed by exposure to a green LED (14.34 J cm(-2)). After treatment, the yeasts recovered from the mice were quantified (CFU/mL) and analyzed for the effects of PDT on their adherence to Buccal epithelial cells. | Photodynamic therapy exhibited antifungal effects against C. albicans biofilms formed in vivo and reduced the capacity of C. albicans to adhere to BECs in vitro |
| Sobaniec et al., [22] | 23 patients aged 31-82 included in the study with oral lichen planus(OLP) | Chlorin e6 (Photolon®), containing 20% chlorin e6 and 10% dimethyl sulfoxide was used as a photosensitizer. A semiconductor laser, with power up to 300 mW and a wavelength of 660 nm. A series of illumination sessions was conducted with the use of superficial light energy density of 90 J/cm2. | The sizes of clinical OLP lesions exposed to PDT were reduced significantly (on average by 55 %). The best effects were observed for the lesions on the lining mucosa (57.6 %). |
CONCLUSION
In conclusion, PDT is a useful modality for the treatment of head and neck tumours and precancerous lesions which present in forms or under conditions that pose considerable difficulties in their management by conventional approaches.
[1]. Dennis E.J., Dolmans G.J., Fukumura D., Jain R K, Photodynamic therapy for cancerNature Reviews Cancer 2003 3:380-87. [Google Scholar]
[2]. Raab O, Ueber die Wirkung fluorescirender Stoffe auf InfusorienZ. Biol 1900 39:524-46. [Google Scholar]
[3]. Nayak CS, Photodynamic theory in dermatologyIndian J Dermatol venereol leprol 2005 71(3):155-60. [Google Scholar]
[4]. Dougherty TJ, Kaufman JE, Goldfarb A, Kenneth R, Weishaupt DB, Mittleman A, Photoradiation therapy for the treatment of malignant tumorsCan Res 1978 38:2628-35. [Google Scholar]
[5]. Macdonald IJ, Dougherty TJ, Basic principles of photodynamic therapyJ Porphyr Phthalocya 2001 5:105-21. [Google Scholar]
[6]. Castono Ana P, Tatiana N D, Mechanisms in photodynamic therapy: part one photosensitizers, photochemistry and cellular localizationPhotodiagnosis Photodyn Ther 2004 1(4):279-93. [Google Scholar]
[7]. Grant WE, Hopper C, MacRobert AJ, Speight PM, Bown SG, Photodynamic therapy of oral cancer with systemic photosensitizationThe Lancet 1993 342:147-48. [Google Scholar]
[8]. Sternberg E, Dolphin D, Bruckner C, Porphyrin-based photosensitizers for use in photodynamic therapyTetrahedron 1998 54:4151-4202. [Google Scholar]
[9]. Kudinova NV, Berezov TT, Photodynamic therapy: search for ideal photosensitizersBiomed. Khim 2009 55(5):558-69. [Google Scholar]
[10]. Panjehpour M, Overholt BF, Haydek JM, Light sources and delivery devices for photodynamic therapy in the gastrointestinal tract Gastrointest EndoscClin N Am 2000 10(3):513-32. [Google Scholar]
[11]. Star W M, Light delivery and light dosimetry for photodynamic therapyLasers Med Sci 1990 5(2):107-13. [Google Scholar]
[12]. Yu CH, Lin HP, Chen HM, Yang H, Wang YP, Chiang CP, Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light emitting diode or laser lightLasers Surg Med 2009 41:628-33. [Google Scholar]
[13]. Lin HP, Chen HM, Yu CH, Yang H, Wang YP, Chiang CP, Topical photodynamic therapy is very effective for oral verrucous hyperplasia and oral erythroleukoplakiaJ Oral Pathol Med 2010 39:624-30. [Google Scholar]
[14]. Grant WE, Hopper C, Speight PM, Macrobert AJ, Bown SG, Photodynamic therapy of malignant and premalignant lesions in patients with ‘field cancerization’ of the oral cavityJ Laryngol Otol 1993 107(12):1140-5. [Google Scholar]
[15]. Fan KF, Hopper C, Speight PM, Buonaccorsi G, Macrobert AJ, Bown SG, Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity cancerCancer 1996 78(7):1374-83. [Google Scholar]
[16]. Kulapaditharom B, Boonkitticharoen V, Photodynamic therapy in management of head and neck cancers and precancerous lesionsJ Med Assoc Thai 2000 83(3):249-58. [Google Scholar]
[17]. Schweitzer VG, Photofrin-mediated photodynamic therapy for treatment of early stage oral cavity and laryngeal malignanciesLasers Surg Med 2001 29(4):305-13. [Google Scholar]
[18]. Lorenz KJ, Maier H, Photodynamic therapy with meta-tetrahydroxyphenylchlorin (Foscan) in the management of squamous cell carcinoma of the head and neck: experience with 35 patientsEur Arch Otorhinolaryngol 2009 266(12):1937-44. [Google Scholar]
[19]. Schweitzer VG, Somers ML, Photofrin-mediated photodynamic therapy for treatment of early stage (tis-t2n0m0) SqCCa of oral cavity and oropharynxLasers Surg Med 2010 42(1):1-8. [Google Scholar]
[20]. Hsu YC, Yang DF, Chiang CP, Lee JW, Tseng MK, Successful treatment of 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch precancerouslesions by topical 5-aminolevulinic acid-mediated photodynamic therapyPhotodiagnosis Photodyn Ther 2012 9(4):310-8. [Google Scholar]
[21]. Costa AC, Campos Rasteiro VM, da Silva Hashimoto ES, Araújo CF, Pereira CA, Junqueira JC, Effect of erythrosine- and LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cellsOral Surg Oral Med Oral Pathol Oral Radiol 2012 114(1):67-74. [Google Scholar]
[22]. Sobaniec S, Bernaczyk P, Pietruski J, Cholewa M, Skurska A, Dolinska E, Clinical assessment of the efficacy of photodynamic therapy in the treatment of oral lichen planusLasers Med Sci 2013 28(1):311-6. [Google Scholar]