A Non-Invasive Technique which Demonstrates the Iron in the Buccal Mucosa of Sickle Cell Anaemia and Thalassaemia Patients who Undergo Repeated Blood Transfusions
Harika Chittamsetty1, M.S. Muni Sekhar2, Syed Afroz Ahmed3, Charu Suri4, Sridevi Palla5, S. Muni Venkatesh6, Shahela Tanveer7
1 Senior Lecturer, Department of Oral Pathology, Sri Sai College of Dental Sugery, Vikarabad, Andhra Pradesh, India.
2 Professor & Head, Department of Oral Pathology, SVS Dental College, Mehboobnagar, Andhra Pradesh, India.
3 Professor & Head, Department of Oral Pathology, Sri Sai College of Dental Sugery Vikarabad, Andhra Pradesh, India.
4 Reader, Department of Oral Pathology, Sri Sai College of Dental Sugery, Vikarabad, Andhra Pradesh, India.
5 Senior Lecturer, Department of Oral Pathology, Panineeya Dental College, Hyderabad, Andhra Pradesh, India.
6Armed Dental Forces, Hyderabad, Andhra Pradesh, India.
7 Senior Lecturer, Department of Oral Pathology, Sri Sai College of Dental Sugery, Vikarabad, Andhra Pradesh, India.
NAME, ADRES, E-MAIL ID OF THE CORESPONDING AUTHOR: Dr. Harika Chittamsetty, Senior Lecturer, Department of Oral Pathology, 1/394, C.S. Buildings, Malavyanagar, Gudur, Nellore District, Andhra Pradesh, India.
Phone: 9966301981
E-mail: drharika17@gmail.com
Background: Iron is vital for all the living organisms. However, excess iron is hazardous because it produces free radical formation. Therefore, the iron absorption is carefully regulated to maintain an equilibrium between the absorption and the body loss of iron. Considering the lack of specific excretory pathways for iron in humans, an iron overload in the tissues is frequently encountered. It can be precipitated by a variety of conditions such as increased iron absorption, as is seen in haemochromatosis or a frequent parenteral iron administration, as is seen in thalassaemia and sickle cell anaemia patients (a transfusional overload).
Objectives: To demonstrate the iron overload at an early stage by oral exfoliative cytology in the oral mucosal cells of thalassaemia and sickle cell anaemia patients and to compare the presence of iron in the exfoliated oral epithelial cells with that of the serum ferritin levels in those patients.
Materials and Methods: The present study comprised of 40 β– thalassaemia major and 20 sickle cell anaemia patients who were undergoing repeated blood transfusions of a minimum of 15/more, along with 60 clinically healthy individuals. Scrapings were obtained from the buccal mucosa and they were smeared onto glass slides. Then the slides were stained with a Perl’s Prussian staining kit and they were examined under a light microscope.
Results: 72.5% of the thalassaemia patients and 35% of the sickle cell anaemia patients revealed a positivity for the Perl’s Prussian blue reaction and none of the controls showed this positivity. It was also observed that as the serum ferritin levels increased, the iron overload in the oral mucosal cells of the thalassaemia patients also increased, which was not statistically significant, whereas it was statistically significant in case of the sickle cell anemia patients.
Conclusion: Since the exfoliative cytology is a simple, painless, non-invasive and a quick procedure to perform, a lot of research should be carried out on the correlation of the Perl’s Prussian blue reaction to the serum ferritin levels.
Iron, Thalassaemia, Sickle cell anaemia, Perl’s Prussian blue reaction
INTRODUCTION
Minerals are inorganic elements that are essential for life and they provide both the structural and regulatory functions of the body. It has been observed that there are at least 29 different minerals in our body, which constitute about 4% of the body weight [1].
Among them, iron is an essential nutrient which is required by every human cell. Approximately 4.5g of iron can be found in an average adult man, most of which is contained in the haemoglobin molecule and in other haem containing proteins. Under normal circumstances, iron is absorbed by the human body at the rate of 1 mg per day. The absorbed iron is distributed for storage, transport and enzymatic functions. Iron is stored in the form of ionic compounds in the body tissues [2,3].
Considering the lack of specific excretory pathways for iron in humans, an iron overload in the tissues is frequently encountered. It can be precipitated by a variety of conditions such as increased iron absorption, as is seen in haemochromatosis or a frequent parenteral iron administration, as is seen in thalassaemia and sickle cell anaemia patients (transfusional overload) [4,5].
The iron accumulation in the β-thalassaemia major patients depends upon the number of blood transfusions which are given. One unit of blood contains 250 ml of RBCs. Since 1ml of RBC contains 1mg of iron, 4 units of blood will contain around 1gm of iron. The signs of a clinical toxicity become apparent, when the body iron concentration reaches 400 to 1000mg/kg of the body weight. The signs of an iron overload can be usually seen after 10-12 transfusions [6].
A liver biopsy is a widely followed, definitive test which is used for assessing the parenchymal iron overload. A bone marrow biopsy is indicated in an iron overload which is associated with the reticuloendothelial system. These procedures are however invasive and they may not be feasible in every occasion.
Oral exfoliative cytology is a simple, painless, bloodless and a quick procedure that involves the study of the exfoliated oral mucosal cells. As the oral exfoliated cells can reflect the changes in the parent tissue, they can be assessed both quantitatively and qualitatively to identify the pathologic alterations, if any, which are associated with the parent tissue. This principle is applied to demonstrate the iron in the oral exfoliated cells of the iron overload patients by using the Perl’s Prussian blue reaction [7,8].
AIMS AND OBJECTIVES
To demonstrate the iron in the oral epithelial cells in patients who suffer from Thalassaemia and Sickle cell anaemia and to compare the presence of the iron in the exfoliated oral epithelial cells with that of the serum ferritin levels in patients with thalassaemia and sickle cell anaemia, who undergo repeated blood transfusions.
MATERIAL AND METHODS
The present study comprised of 40 β-thalassaemia major (29 Males, 11Females, M:F=29:11) and 20 Sickle cell anaemia patients (15 Males, 05Females, M:F=15:5) who were undergoing repeated blood transfusions of a minimum of 15/more times, who were in the age group of 04-24 years. They were selected through a convenience sampling technique from among the patients who reported to the Thalassemia Sickle cell Society, Hyderabad, for a duration of 1 year. The procedures which were followed were in accordance with the ethical standards of the Institutional Review Committee. The control group consisted of 60 clinically healthy individuals, irrespective of their age and sex (age and sex are non determinants with regards to the demonstration of the iron overload) and the study duration was 1 year. Both the control and the study groups excluded the persons with iron deficiency anaemia, megaloblastic anaemia, malignancy and chronic liver damage.
The materials which were used were a mouth mirror, a wooden spatula, glass micro slides, a fixative solution (70% ethanol), Coplin jars to carry the slides and a Perl’s Prussian staining kit.
For the preparation of the smears, clean, fresh and dry glass slides were used. The patients from the study group and the control group were asked to gargle their mouth with distilled water. Scrapings were obtained from their mouths by using a wet wooden spatula which was moistened with normal saline. By using a gentle scraping motion and by exerting little pressure, cells were scraped from the apparently normal buccal mucosa of both the study and the control groups. The scraps were smeared onto the centre of glass slides and they were spread over a large area to prevent clumping of the cells. From each subject, two smears were prepared. The slides were immediately fixed in 70% ethanol for one hour, to ensure a proper fixation and they were then stained with the Perl’s Prussian staining kit which consisted of Potassium ferrocyanide, which would react with the ferritin in the cells to form a blue colour. Blue granules indicated the presence of an iron overload. The stained smears were examined under a light microscope through the transmitted light at 4X, 10X and 100X magnifications, to study the presence or absence of blue coloured granules in the cells.
RESULTS AND OBSERVATIONS
Among the 40 (100%) thalassaemia patients, 29(72.5%) were iron positive and 11(27.5%) were iron negative for the Perl’s Prussian blue reaction [Table/Fig-1,2]. Among the 20 (100%) sickle cell anaemia patients, 7(35%) were iron positive and 13(65%) were iron negative for the Perl’s Prussian blue reaction [Table/Fig-3,4]. Out of the 60 controls, none showed positivity. On applying the Chi-square test to compute the p-value, from the computations, it was evident that p was <0.05 [Table/Fig-5,6]. Hence, the distribution of the iron overload in both the thalassaemia and the sickle cell anaemia patients was statistically significant.
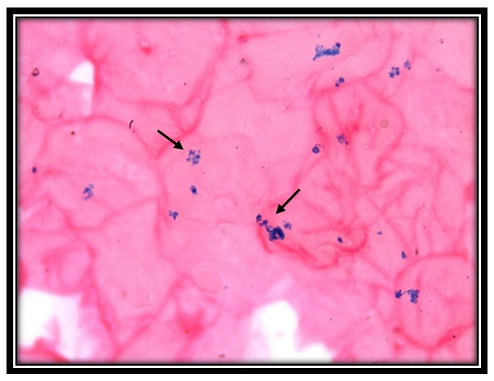
Iron positivity for perl’sprussian stain in oral mucosal cells of thalassemia patient (10x)
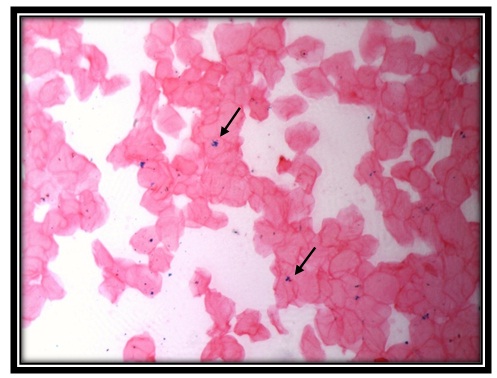
Iron positivity for perl’sprussian stain in oral mucosal cells of thalassemia patient (40X)
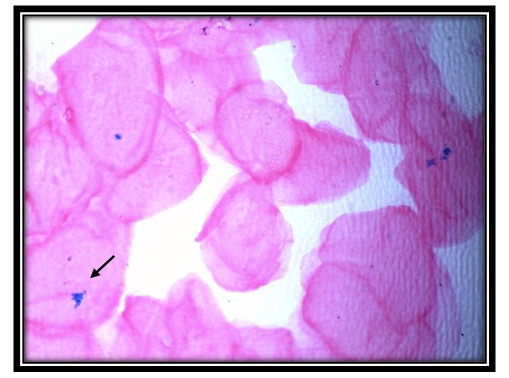
Iron positivity for perl’sprussian stain in oral mucosal cells of sickle cell anemia patient (10x)
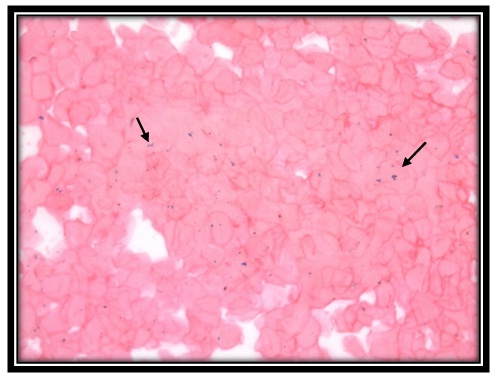
Ironpositivity for perl’sprussian stain in oral mucosal cells of sickle cell anemia patient (40x)
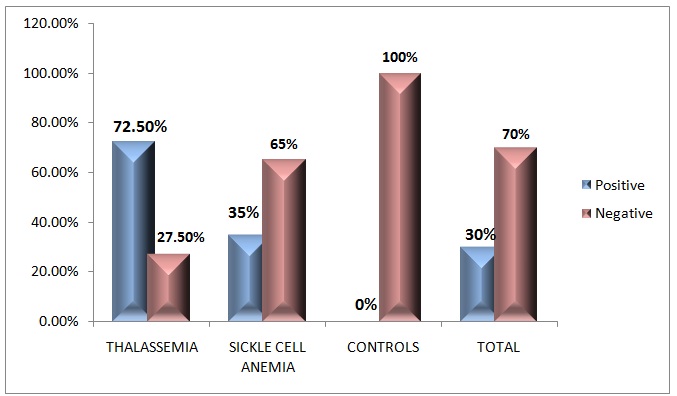
Distribution of iron in oral epithelial cells of thalassemia, sickle cell anemia patients and controls
| Group | Iron Overload in Oral Mucosal Cells | Total |
|---|
| Positive | Negative |
|---|
| Thalassemia | 29(72.5%) | 11(27.5%) | 40(100%) |
| Sickle Cell Anemia | 07(35%) | 13(65%) | 20(100%) |
| Controls | 0(0%) | 60(100%) | 60(100%) |
| Total | 36(30%) | 84(70%) | 120(100%) |
Chisquare value: 60.4, (p<0.05)***
Distribution of iron in oral epithelial cells of thalassemia and sickle cell anemia patient
The correlation of the presence of iron in the exfoliated oral epithelial cells with that of the serum ferritin levels in the patients with thalassaemia and sickle cell anaemia was done. On applying the Mann-Whitney U Test (non-parametric test) to compute the p-value, from the computations, it was evident that p was >0.05 in the thalassaemia patients, which was statistically non significant and that p was <0.05 in the sickle cell anaemia patients, which was statistically significant [Table/Fig-7].
Correlation of presence of iron in exfoliated oral epithelial cells with that of serum ferritn levels in patients with thalassemia and sickle cell anemia
| Group | Iron in Oral Mucosal Cells | No. of Cases | Mean of Serum Ferritin Levels | Standard Deviation of Serum Ferritin Levels | P Value |
|---|
| Thalassemia | Serum ferritin | Positive | 29 | 2094.00 | 1709.351 | p>0.05 |
| Negative | 11 | 1468.55 | 1531.310 |
| Sickle cell anemia | Serum ferritin | Positive | 7 | 1842.86 | 1621.560 | p<0.05** |
| Negative | 13 | 573.08 | 871.860 |
(Mann-Whitney U Test)
DISCUSSION
It is well known that an excess of iron in the tissues is toxic. The toxic effects of the chronic iron deposition in the tissues have been recognized for many years in the idiopathic haemochromatosis and in the transfusion haemosiderosis conditions of thalassaemia and sickle cell anaemia. There are several methods for assessing the body iron overload, like liver biopsies, bone marrow biopsies, etc. However, these procedures are invasive and are not feasible on all occasions. Oral exfoliative cytology is a simple, noninvasive and a safe procedure for estimating the iron overload by using the Perl’s Prussian blue stain [9–11].
The Perl’s Prussian blue reaction is considered to be a classical histochemical reaction which is carried out and is widely applied in the field of haematology. The treatment with a dilute acid is an essential preliminary step in the performance of the Prussian blue reaction. By means of this, the ferric iron is liberated from the unreactive loose combinations with proteins such as haemosiderin. In our study, this technique was applied to the exfoliated buccal mucosal cells, considering the fact that the exfoliated cells possibly represent the changes in the underlying parent tissue. As no previous data is available on the Perl’s Prussian blue positivity in the exfoliated cells, the positivity for the reaction in our study group was compared with the Perl’s reaction positivity in iron overload tissues like liver biopsies [7,8].
In the present study, cytological smears from the exfoliated cells of the buccal mucosa of thalassaemia patients who were undergoing repeated blood transfusions were stained with the Perl’s Prussian blue technique to demonstrate the presence of an iron overload. Out of 40 thalassaemia patients, 29 patients (72.5%) showed iron positivity in the Perl’s Prussian blue reaction, which was statistically significant (p<0.05) as compared to those in the sickle cell anaemia patients and in the controls. Hence, we are of the opinion that the oral epithelial cells can reflect the changes in the liver tissues with an iron overload. This shows a strong association between the thalassaemia patients who were undergoing repeated blood transfusions and an oral mucosal iron overload.
The findings of this study were in accordance with the findings of the study done by Gururaj and Sivapathasundaram (2004) on ten patients, which revealed a positivity for the Perl’s Prussian blue reaction in all the ten cases. The present study was also similar to the study which was done by S. Nandaprasad and P. Sharada (2010), in which the exfoliated cells from the buccal mucosa in 65 of the 100 thalassaaemia patients revealed a positivity for the Perl’s Prussian blue reaction. In addition, in all the three studies (that of Gururaj and Sivapathasundaram, that of S. Nandaprasad and P. Sharada and our present study), none of the patients in the control group showed positivity for the Perl’s Prussian blue reaction.
In the present study, out of the 20 sickle cell anaemia patients, only 7 patients (35%) showed iron positivity in the Perl’s Prussian blue reaction. This might be due to the less number of blood transfusions which the patients had undergone, which had probably resulted in a lesser iron overload in the oral mucosal cells. As no previous data is available on estimation of the iron overload in the oral mucosal cells of the sickle cell anaemia patients who were undergoing repeated blood transfusions, much more research should be carried out on a larger number of samples.
In the present study, an attempt was made to compare the presence of iron in the exfoliated oral epithelial cells with that of the serum ferritin levels in the patients with thalassaemia and sickle cell anaemia, who were undergoing repeated blood transfusions. It was observed that as the serum ferritin levels increased, the iron overload in the oral mucosal cells of the thalassaemia patients also increased, which was not statistically significant, whereas it was statistically significant in case of the sickle cell anaemia patients. This might be due to the small sample size and also the wide range of variations in the serum ferritin levels (215ng/ml - 6990ng/ml) which were observed in the thalassaemia patients as compared to those in the sickle anaemia patients (100ng/ml - 1420ng/ml). Hence, further studies on the correlation of the Perl’s Prussian blue reaction with the serum ferritin levels have to be carried out on a larger number of samples.
The present study was a qualitative study which determined only the presence or absence of an iron overload in the oral mucosal cells of thalassaemia and sickle cell anaemia patients, but the amount of the iron overload cannot be measured quantitatively. Carrying out studies with larger sample sizes and a quantitative assessment of the iron overload can probably establish this non-invasive procedure as an ideal screening and diagnostic tool in all the patients who undergo repeated blood transfusions.
CONCLUSIONS
The objective of this study was to establish oral exfoliative cytology as an ideal screening and diagnostic tool in thalassaemia and sickle cell anaemia patients who undergo repeated blood transfusions. Owing to the smaller sample size and the lack of a correlative Perl’s staining in the tissue biopsies in our cases, the diagnostic reliability of the Perl’s Prussian blue reaction in the oral exfoliated cells, for the demonstration of an iron overload, is still debatable.
By considering the simplicity and the acceptability of the exfoliative cytology methods, further studies which correlate the Perl’s Prussian blue reaction with the serum ferritin levels and the MRI images can establish this non-invasive procedure as an ideal screening and diagnostic tool in all the patients who undergo repeated blood transfusions and they can also assess the future complications which are associated with an iron overload and thereby help in the institution of the appropriate treatment.
Chisquare value: 60.4, (p<0.05)***
(Mann-Whitney U Test)
[1]. Satyanarayana U, Biochemistry 2001 First editionArunabha Sen:457-59. [Google Scholar]
[2]. Young IS, Woodside JV, Antioxidants in health and diseaseJ Clin Pathol 2001 54:176-86. [Google Scholar]
[3]. Conrad Marcel E, Numbrelt Jay, Iron Absorption and Transport – An UpdateAm. J. Hematol 2000 64:287-98. [Google Scholar]
[4]. Hoffbrand AV, Pettit JE, Moss PAH, Essential Haematology 2005 Fourth EditionBlackwell Publishing Company:28-41. [Google Scholar]
[5]. Andrews Nancy C, Disorders of Iron MetabolismThe New England Journal of Medicine 1999 23:1986-95. [Google Scholar]
[6]. Kumar Vinay, Cotran Ramzi S, Robbins Stanley L, Basic Pathology 1997 Sixth EditionHarcourt Asia PTE. LTD:353-54. [Google Scholar]
[7]. Gururaj N, Sivapathasundaram B, Demonstration of iron in the exfoliated cells of oral mucosaJOMFP 2003 7(2):37-39. [Google Scholar]
[8]. Nandaprasad S, Sharada P, Vidya M, Karkera B, Hemanth M, Prakash N, Oral Exfoliative Cytology In Beta Thalassaemia Patients Undergoing Repeated Blood TransfusionsThe Internet Journal of Pathology 2009 10(1) [Google Scholar]
[9]. Beutler Ernest, Hoffbrand A Victor, Cook James D, Iron Deficiency and OverloadHematology 2003 [Google Scholar]
[10]. Smith R Sephton, Iron Deficiency and Iron OverloadArch. Dis. Child 1965 40:343-63. [Google Scholar]
[11]. Vermyle Christiane, What is new in iron overload?Eur J Pediatr 2008 167:377-81. [Google Scholar]